The role of technetium Tc99m-tetrofosmin as head and neck tumor-seeking agent: a preliminary report
Abstract
Aim: To evaluate the diagnostic value of technetium Tc99m-tetrofosmin (99mTc-TF) in primary cancers of the head and neck. Methods: Methods: Single photon emission computer tomography with planar imaging of the neck for primary site evaluation and whole body scanning for assessment of metastases in 12 patients with newly diagnosed head and neck cancer. Tumor-to-background index (T/Bg) was derived in patients with positive findings (tumor or lymph nodes). Results: The tomographic images showed increased tracer uptake in pathological sites (primary tumor or lymph node) in 9 patients (overall sensitivity 75%). Primary tumor was visualised in 7 patients (sensitivity 58%) and infiltrated lymph nodes in 4 out 7 patients (sensitivity 57%). Mean values for T/Bg index were 5.44 ± 1.28 for primary tumor and 4.25 ± 1.67 for lymph nodes. Mean values for T/Bg index were 4.5 ± 0.71 for patients with in situ and grade I carcinoma and 6.68 ± 0.36 for patients with tumor grade II and III (P = 0.034, Mann-Whitney U test). Conclusion: The present study demonstrates that 99mTc-TF is a valuable radiotracer for head and neck cancer imaging. To determine the potential role of this imaging protocol in clinical practice will require a larger sample size.
Keywords
Introduction
Malignant tumors of the head and neck are among the six most common forms of tumors in the body. Head and neck cancer include neoplasms of the upper respiratory tract (nasopharynx, oropharynx, larynx) salivary glands, and soft tissue of the neck with squamous cell carcinoma being the most frequent histological type. Diagnosis of this type of cancer is based on endoscopy and biopsy of the suspicious lesion.[1] Staging and evaluation of the extent of disease involves the use of computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET).[2] Follow-up evaluations after surgery and/or radiotherapy and differential diagnosis of disease relapse from radiation necrosis is more difficult to assess, due to extensive distortion of the normal anatomy, and may require a combination of different imaging tests,[3-7] such as PET scan.[8-11] However, its availability is limited by the need for a cyclotron that produces 18Fluorodeoxyglucose (18FDG) and its increased cost. In addition, in many countries, PET is not widely available so other methods are needed for tumor-imaging assessment.
When a PET scanner is not available, nuclear medicine still plays a crucial role in the initial staging of the disease and for follow ups. Lymph node cancerous involvement at initial presentation may be difficult to assess by conventional imaging methods based only on morphology and size. Functional nuclear medicine imaging has the unique advantage of assessment of the metabolic state of lymph nodes. Currently, the agents employed are Thalium-201 (201Tl) and the two technetium-99m labelled compounds Sestamibi (MIBI) and Tetrofosmin.[2]
99mTc-MIBI and Tc99m-tetrofosmin (99mTc-TF) are two lipophilic cationic complexes, which were originally employed in myocardial perfusion imaging, but later were found to possess tumor-seeking properties in the evaluation of diverse human malignancies.[12] The diagnostic value of 99mTc-TF could hold promise as a head and neck cancer tracer, although limited data exist in clinical research.[13,15] In the present study the diagnostic utility of 99mTc-TF prior to surgery of head and neck neoplasms was assessed and correlated with the 99mTc-TF uptake of histological grade, and tumor and lymph node size.
Methods
Prior to surgery, 12 subjects (11 males and 1 female) of median age 65.5-year-old (48-83) with suspected head and neck cancer had 99mTc-TF planar and Single photon emission computer tomography (SPECT) imaging of the neck and whole body scanning for metastatic evaluation. None of the patients had received treatment prior to scintigraphy, except for patient twelve, who had surgery, radiotherapy and chemotherapy, one year prior to scintigraphy. This patient had scintigraphy because of a tumor relapse at the primary tumor.
All patients were interviewed before 99mTc-TF imaging and the patients’ age, height, weight, smoking habits, and alcohol consumption were noted. Data from the CT findings included the anatomical location and size of the tumor, as well as the existence and size of lymph nodes. Correlation of SPECT imaging with the tumor histopathology diagnosis and grade was performed after the original tumor was excised and the pathology was established. The protocol was approved by the Hospital Research and Ethics Committee. Informed consent was obtained from all patients. Clinical trial registration was not required for this small study.
Protocol
All patients were injected with a dose of 740 MBq (20 mCi) bolus 99mTc-TF; and immediately after injection patients drank 5 mL of lemon juice to stimulate salivary glands. Lemon juice stimulation achieves the lowest possible uptake of radiotracer in the salivary glands (normal distribution of 99mTc-TF) during imaging. Scintigraphy was performed with patients in a supine position. Anterior planar images were acquired 5-10 min post injection, using a zoom factor of 2.66. Tomography was acquired 15 min post-injection with the dual-head camera at 6o-angles (60 stops) and 30 s per projection (30 projection/head) over a 360° arc, using a low-energy, general purpose collimator. Acquisition was obtained with a matrix size of 64 × 64 × 16, 1.85 zoom factor, and a 15% symmetric window at 140 keV (no contour). Reconstruction method was filtered back projection (filter butterworth, cut-off frequency 0.5, power 7.0). No attenuation correction was used. Finally, whole body scan was acquired in all patients for possible distant metastases evaluation.
Two nuclear medicine specialists visually evaluated the planar, tomographic and whole body images, which were compared to the CT scans. Increased uptake in SPECT images (in a site of pathological finding on CT (primary tumor or lymph node) was considered a positive finding. A region of interest (ROI) was drawn on the relative coronal image. Background (Bg) ROI was drawn over the apex of the right lung. T/Bg index for tumor and lymph nodes was derived in all patients with positive findings. Patients with no significant uptake on pathological sites were considered negative.
Statistical analysis
Data are presented as mean (± standard deviation).Uptake of 99mTc-TF (T/Bg index) in tumors, as well as sensitivity, was correlated to tumor histological grade, and the tumor and regional lymph node size. For statistical analysis, the software “SPSS for Windows” (P ≤ 0.05 was considered as statistically significant). Non parametric statistics was applied. The Mann-Whitney U test compared means and the chi-square test to compare frequencies.
Results
The characteristics of the twelve patients and the tumors are summarized in [Table 1].
Physical characteristics of the patients and tumor anatomical location
| Characteristics | Patients |
|---|---|
| Gender | 11 M/1 F |
| Mean age (years) | 65.75 ± 11.8 (48-83) |
| Height (cm) | 166.75 ± 8.9 (155-184) |
| Weight (kg) | 69.25 ± 9.5 (55-82) |
| BMI (kg/m2) | 25.00 ± 3.8 (16.2-32.4) |
| Smoking | 11/12 (91.7%) |
| * 0: 1/12 (8.3%) | |
| * 1: 0/12 (0.0%) | |
| * 2: 11/12 (91.7%) | |
| Alcohol | 8/12 (66.7%) |
| ** 0: 4/12 (33.3%) | |
| ** 1: 2/12 (16.7%) | |
| ** 2: 6/12 (50.0%) | |
| Anatomical location of tumor | |
| Eepiglottis | 1/12 (8.3%) |
| Vocal cord | 2/12 (16.7%) |
| Base of tongue | 2/12 (16.7%) |
| Pharyngeal wall | 1/12 (8.3%) |
| Tonsil | 1/12 (8.3%) |
| Submandibular gland | 1/12 (8.3%) |
| Soft palate | 1/12 (8.3%) |
| Pyriform fossa | 2/12 (16.7%) |
| Nasopharynx | 1/12 (8.3%) |
| Histopathological findings | |
| Squamous cell | 10/12 (83.3%) |
| Adenoid cystic cell | 2/12 (16.7%) |
Among them, 4 patients had in situ carcinomas, 2 patients grade I carcinoma, 1 patient grade II and 5 patients grade III carcinoma. The smallest measurable tumor was 2.0 cm, and the largest 5.5 cm. Pathological enlarged lymph nodes were noted in 7 patients with sizes between 2 cm and 5 cm [Table 2].
Tumor and regional lymph node characteristics in patients with head and neck cancer
| Patient | Tumor diameter: cm (max) | LN diameter: cm (max) | T/Bg Index | LN/Bg index | Histological Grade* |
|---|---|---|---|---|---|
| 1 | 3 | 5 | 5.2 | 5.18 | I |
| 2 | 2.5 | No LNs | 3.52 | No LNs | 0 |
| 3 | 2 | No LNs | 4.57 | No LNs | 0 |
| 4 | 4.5 | 4.5 | 4.72 | 3.17 | I |
| 5 | 2 | 2 | - | - | 0 |
| 6 | 3 | 3 | 6.44 | - | III |
| 7 | 2.5 | 2.5 | - | 2.55 | III |
| 8 | 2.5 | 4 | - | 6.1 | II |
| 9 | 6 | No LNs | 6.5 | No LNs | III |
| 10 | 4.5 | 2 | - | - | III |
| 11 | 2.5 | No LNs | - | No LNs | 0 |
| 12 | 5.5 | No LNs | 7.1 | No LNs | III |
Tomographic images showed increased tracer uptake in pathological sites (primary tumor or lymph nodes) in 9 patients, with an overall disease detection sensitivity of 75% [Figure 1]. Primary tumor was visualised in seven patients (sensitivity 58%) and infiltrated lymph nodes in 4 out of 7 patients (sensitivity 57%) [Table 3]. According to histological tumor grade, 4 (66.7%) patients with in situ or grade I and 3 (50%) patients with grades II or III had higher 99mTc-TF tumor uptake (P = 0.373) than the counterparts. Scintigraphic sensitivity was lower with tumor size < 3 cm (33.3%) compared to tumor size ≥ 3 cm (83.4%) sensitivity. The statistical analysis found a trend towards a positive correlation with tumor size in radiotracer uptake, although the results did not reach statistical significance (P = 0.079) [Table 3]. In addition, one patient with tumor size of 4.5 cm had no uptake [Table 2]. Among the 7 patients with 99mTc-TF uptake in primary tumor, 4 patients exhibited increased 99mTc-TF uptake in the regional infiltrated lymph nodes. One of them had lymph node size < 3, while the other patients had lymph node sizes larger than 3 cm. Two of the 5 patients with no primary tumor uptake had uptake in the lymph nodes [Table 2].
Tumor and lymph node uptake of 99mTc-TF and uptake sensitivity according to tumor grade and size and lymph node size in patients with head and neck cancer
| Patients | Patients No. | TF uptake sensitivity (%) | P | Mean T/Bg ± SD | P |
|---|---|---|---|---|---|
| In situ and Grade I | 4/6 | 66.7% | 0.343 | 4.05 ± 0.71 | 0.034* |
| Grade II and III | 3/6 | 50.0% | 6.68 ± 0.66 | ||
| Total | 7/12 | 75.0% | 5.44 ± 1.28 | ||
| Tumor size < 3cm | 2/6 | 33.3% | 0.074 | 4.05 ± 0.74 | 0.053 |
| Tumor size ≥ 3cm | 5/6 | 83.4% | 5.99 ± 0.99 | ||
| Total | 7/12 | 75.0% | 5.44 ± 1.28 | ||
| LN size < 3 cm | 1/3 | 33.3% | 0.32 | 2.55 | 0.180 |
| LN size ≥ 3 cm | 3/4 | 75.0% | 3.99 ± 1.5 | ||
| Total | 4/7 | 57.0% | 4.25 ± 1.67 |
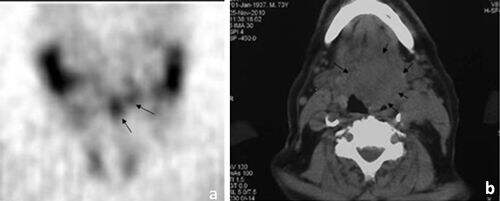
Figure 1. Patient No. 4 with base of tongue cancer: (a) SPECT coronal slice (arrows showing the 99mTc-TF uptake); (b) relevant CT slice (arrows showing the tumor)
Mean values for T/Bg index in all patients was 5.44 ± 1.28 for primary tumor and 4.25 ± 1.67 for lymph nodes. Statistical difference was found with histological grade, after categorizing the patients according to their grade, tumor and lymph node size. Thus, concerning histological grade, patients with tumor grades 0 or I had mean values for 4.5 ± 0.71, whereas patients with tumor grades II or III had T/Bg indexes of 6.68 ± 0.36. Statistically significant difference between the 2 groups was found (P = 0.034, Mann-Whitney U test) [Table 3]. Regarding tumor size, T/Bg index was lower in tumors < 3 cm (4.05 ± 0.74) than in tumors ≥ 3 cm (5.99 ± 0.99). After statistical analysis, there was a trend towards a positive correlation of T/Bg index with increasing tumor size (P = 0.053) [Table 3]. Mean values for T/Bg index of lymph nodes < 3 cm were 2.55, and in lymph nodes ≥ 3 cm, was 3.99 ± 1.5. There was no statistical difference (P = 0.180), possible due to the small number of cases [Table 3]. No metastatic lesions were found on whole body images.
Discussion
The study showed as sensitivity of SPECT in pathological sites (either primary tumor or regional lymph nodes) of 75%. SPECT sensitivity for only primary tumor diagnosis was 58% while for infiltrated lymph nodes it was 57%. In accordance with our findings, a previous study in 10 patients with nasopharyngeal carcinoma reported the 99mTc-TF uptake in 7 out of 10 patients (70%).[15] Fattori et al.[14] studied exclusively patients with laryngeal cancer using 99mTc-TF and reported 96% sensitivity for detecting the primary mass and 50% for lymph node involvement. Variations of sensitivities using 99mTc-TF uptake in primary cancers of the head and neck between studies may be caused by a small study sample, but it may be higher in patients with exclusively laryngeal cancer according to other trials.[14]
In another study of 21 patients with nasopharyngeal carcinoma that evaluated 99mTc-MIBI, sensitivity was 97% and specificity 100%.[16] A study that compared 99mTc-MIBI to 99mTc-TF in nasopharyngeal cancers found that both radiotracers detected all primary tumors, 99mTc-MIBI was superior in detecting pathological lymph nodes (sensitivity 95% vs. 79%).[13] The same authors also reported better sensitivity for 99mTc-MIBI compared to 201Tl during monitoring response to radiotherapy.[17] Similarly, another study reported a limited role of 201Tl in detection of the primary tumor with a sensitivity of 54%, specificity 75% and accuracy 57%.[18]
In contrast, Wang et al.[19] reported that 201Tl was more sensitive than 99mTc-MIBI, with 201Tl SPECT identifying 94% of the primary lesions in head and neck cancers with different sites, and all of the positive and two negative lymph nodes.[20]
Another study found a higher percentages for sensitivity (88%) and specificity (94%).[21] Shiau et al.[22] reported a 64% sensitivity for 99mTc-MIBI in primary tumor detection and 73%[23] for tumor recurrence. Shen et al.[15] reported higher specificity, but lower sensitivity for 99mTc-TF, as compared to CT. Finally, other researchers suggested that both 201Tl and 99mTc-MIBI have the same accuracies in locating primary, recurrent and lymph node involvement and thus could also be valuable.[24]
In the study, no relation of the 99mTc-TF sensitivity with the pathological grade of the tumor was observed, but there was a potential correlation of 99mTc-TF sensitivity with tumor size. Thus, it appeared to be a trend toward positive uptake with increasing tumor size, which did not reach statistical significance, probably a result of a relatively small number of cases. In contrast, 99mTc-TF result from previous studies with 99mTc-MIBI did correlated tumor size, stage, or histology and it did not affect the tracer uptake.[25] Five patients did not have any 99mTc-TF uptake and no tumor was visualized. All but one of these tumors had a size < 3 cm and were in the pharyngeal wall, with close proximity to the tonsils and the pyriform fossa. Lower sensitivity in this area could be due to the complex anatomy and physiological uptake, which make tumor distinction more difficult. Although not verified in the present study, another possibility for negative radiotracer uptake by some tumors could be a possible molecular mechanism that pumps the radiopharmaceutical out of the tumor cells.[26] Such mechanisms attributed to membrane multidrug resistance proteins have been associated with resistance to chemotherapy,[27-29] and or linked to limited or no radiotracer uptake in a variety of tumors.[30-33] if this mechanism proves to be significant in head and neck cancers, then the 99mTc-TF scintigrams may be useful in therapy planning for these patients.
Although the tumor grade was not correlated to radiotracer uptake sensitivity, it was positively correlated to T/Bg index. In another study, false-positive cases were reported when T/Bg index were ≤ 1.7.[13] In our study, no patient with radiotracer uptake had a T/Bg index ≤ 1.7.
In conclusion, the study showed that 99mTc-TF SPECT had an overall sensitivity for visualization of a head and neck primary cancer site of 58%. However, sensitivity was lower for certain tumor locations than others. For example, patients with tonsillar and pyriform fossa tumors didn’t have any uptake (false negative exam). Since these sites are difficult to assess, even with SPECT, the results in these locations should be interpreted with caution. Generally, SPECT should be accurate for visualization of tumors of > 3 cm in any other location. The T/Bg ratio was correlated with malignancy grade. Larger studies will help to increase the statistic power (as well as comparison with 18FDG PET/CT) to establish a role for 99mTc-TF SPECT in therapy, as well as prognosis of head and neck cancers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
2. Vermeersch H, Loose D, Ham H, Otte A, Van de Wiele C. Nuclear medicine imaging for the assessment of primary and recurrent head and neck carcinoma using routinely available tracers. Eur J Nucl Med Mol Imaging 2003;30:1689-700.
3. Manolidis S, Donald PJ, Volk P, Pounds TR. The use of positron emission tomography scanning in occult and recurrent head and neck cancer. Acta Otolaryngol Suppl 1998;534:1-11.
4. Nowak B, Di Martino E, Jänicke S, Cremerius U, Adam G, Zimny M, Reinartz P, Büll U. Diagnostic evaluation of malignant head and neck cancer by F-18-FDG PET compared to CT/MRI. Nuklearmedizin 1999;38:312-8.
5. Pöpperl G, Lang S, Dagdelen O, Jäger L, Tiling R, Hahn K, Tatsch K. Correlation of FDG-PET and MRI/CT with histopathology in primary diagnosis, lymph node staging and diagnosis of recurrency of head and neck cancer. Rofo 2002;174:714-20. (in German)
6. Benchaou M, Lehmann W, Slosman DO, Becker M, Lemoine R, Rufenacht D, Donath A. The role of FDG-PET in the preoperative assessment of N-staging in head and neck cancer. Acta Otolaryngol 1996;116:332-5.
7. Stuckensen T, Kovacs AF, Adams S, Baum RP. Staging of the neck in patients with oral cavity squamous cell carcinomas: a prospective comparison of PET, ultrasound, CT and MRI. J Craniomaxillofac Surg 2000;28:319-24.
8. Hannah A, Scott AM, Tochon-Danguy H, Chan JG, Akhurst T, Berlangieri S, Price D, Smith GJ, Schelleman T, McKay WJ, Sizeland A. Evaluation of 18 F-fluorodeoxyglucose positron emission tomography and computed tomography with histopathologic correlation in the initial staging of head and neck cancer. Ann Surg 2002;236:208-17.
9. Kresnik E, Mikosch P, Gallowitsch HJ, Kogler D, Wiesser S, Heinisch M, Unterweger O, Raunik W, Kumnig G, Gomez I, Grünbacher G, Lind P. Evaluation of head and neck cancer with 18F-FDG PET: a comparison with conventional methods. Eur J Nucl Med 2001;28:816-21.
10. Teknos TN, Rosenthal EL, Lee D, Taylor R, Marn CS. Positron emission tomography in the evaluation of stage III and IV head and neck cancer. Head Neck 2001;23:1056-60.
11. Li P, Zhuang H, Mozley PD, Denittis A, Yeh D, Machtay M, Smith R, Alavi A. Evaluation of recurrent squamous cell carcinoma of the head and neck with FDG positron emission tomography. Clin Nucl Med 2001;26:131-5.
12. Schillaci O, Spanu A, Madeddu G. [99mTc] sestamibi and [99mTc] tetrofosmin in oncology: SPECT and fusion imaging in lung cancer, malignant lymphomas and brain tumors. Q J Nucl Med Mol Imaging 2005;49:133-44.
13. Kostakoglu L, Uysal U, Ozyar E, Demirkazik FB, Hayran M, Atahan L, Bekdik CF. A comparative study of technetium-99m sestamibi and technetium-99m tetrofosmin single-photon tomography in the detection of nasopharyngeal carcinoma. Eur J Nucl Med 1997;24:621-8.
14. Fattori B, Grosso M, Nacci A, Bianchi F, Cosottini M, Ursino F, Manca G, Rubello D, Strauss HW, Mariani G. The role of 99mTc-tetrofosmin scintigraphy for staging patients with laryngeal cancer. Cancer Biother Radiopharm 2005;20:27-35.
15. Shen YY, Kao CH, Changlai SP, Chieng PU, Yen TC. Detection of nasopharyngeal carcinoma with head and neck Tc-99m tetrofosmin SPECT imaging. Clin Nucl Med 1998;23:305-8.
16. Pui MH, Du JQ, Yueh TC, Zeng SQ. Imaging of nasopharyngeal carcinoma with Tc-99m MIBI. Clin Nucl Med 1998;23:29-32.
17. Kostakoglu L, Uysal U, Ozyar E, Hayran M, Uzal D, Demirkazïk FB, Kars A, Atahan L, Bekdik CF. Monitoring response to therapy with thallium-201 and technetium-99m-sestamibi SPECT in nasopharyngeal carcinoma. J Nucl Med 1997;38:1009-14.
18. Guney E, Yigitbasi OG, Tutus A, Bozdemir K, Nardali M. Value of thallium-201 scintigraphy for primary tumour detection in patients with malignant neck masses. Eur J Nucl Med 1998;25:431-4.
19. Wang SJ, Hsu CY, Lin WY, Kao CH, Jan JS, Yeh SH. Comparison of TI-201 and Tc-99m MIBI SPECT imaging in nasopharyngeal carcinoma. Clin Nucl Med 1995;20:800-2.
20. Gregor RT, Valdes-Olmos R, Koops W, Balm AJ, Hilgers FJ, Hoefnagel CA. Preliminary experience with thallous chloride T1 201-labeled single-photon emission computed tomography scanning in head and neck cancer. Arch Otolaryngol Head Neck Surg 1996;122:509-14.
21. Mukherji SK, Gapany M, Phillips D, Neelon B, O'Brien S, McCartney W, Buejenovich S, Parekh JS, Noordzij JP, Castillo M. Thallium-201 single-photon emission CT versus CT for the detection of recurrent squamous cell carcinoma of the head and neck. AJNR Am J Neuroradiol 1999;20:1215-20.
22. Shiau YC, Tsai SC, Ho YJ, Kao CH. Comparison of technetium-99m methoxyisobutylisonitrile single photon emission computed tomography and computed tomography to detect recurrent or residual nasopharyngeal carcinomas after radiotherapy. Anticancer Res 2001;21:2213-7.
23. Kao CH, Shiau YC, Shen YY, Yen RF. Detection of recurrent or persistent nasopharyngeal carcinomas after radiotherapy with technetium-99m methoxyisobutylisonitrile single photon emission computed tomography and computed tomography: comparison with 18-fluoro-2-deoxyglucose positron emission tomography. Cancer 2002;94:1981-6.
24. Sobic-Saranovic DP, Pendjer IP, Kozarevic N, Artiko VM, Mikic AA, Obradovic VB. Evaluation of undifferentiated carcinoma of nasopharyngeal type with thallium-201 and technetium-99m MIBI SPECT. Otolaryngol Head Neck Surg 2007;137:405-11.
25. Leitha T, Glaser C, Pruckmayer M, Rasse M, Millesi W, Lang S, Nasel C, Backfrieder W, Kainberger F. Technetium-99m-MIBI in primary and recurrent head and neck tumors: contribution of bone SPECT image fusion. J Nucl Med 1998;39:1166-71.
26. Nakamura K, Sammiya T, Hashimoto J, Ishibashi R, Matsumoto K, Kubo A. Comparison of cationic myocardial perfusion agents: characteristics of accumulation in cultured smooth muscle cells. Ann Nucl Med 1996;10:375-81.
27. Chiu ML, Kronauge JF, Piwnica-Worms D. Effect of mitochondrial and plasma membrane potentials on accumulation of hexakis (2-methoxyisobutylisonitrile) technetium(I) in cultured mouse fibroblasts. J Nucl Med 1990;31:1646-53.
28. Crankshaw CL, Marmion M, Luker GD, Rao V, Dahlheimer J, Burleigh BD, Webb E, Deutsch KF, Piwnica-Worms D. Novel technetium (III)-Q complexes for functional imaging of multidrug resistance (MDR1) P-glycoprotein. J Nucl Med 1998;39:77-86.
29. Márián T, Szabó G, Goda K, Nagy H, Szincsák N, Juhász I, Galuska L, Balkay L, Mikecz P, Trón L, Krasznai Z. In vivo and in vitro multitracer analyses of P-glycoprotein expression-related multidrug resistance. Eur J Nucl Med Mol Imaging 2003;30:1147-54.
30. Utsunomiya K, Ballinger JR, Piquette-Miller M, Rauth AM, Tang W, Su ZF, Ichise M. Comparison of the accumulation and efflux kinetics of technetium-99m sestamibi and technetium-99m tetrofosmin in an MRP-expressing tumour cell line. Eur J Nucl Med 2000;27:1786-92.
31. Muzzammil T, Moore MJ, Ballinger JR. In vitro comparison of sestamibi, tetrofosmin, and furifosmin as agents for functional imaging of multidrug resistance in tumors. Cancer Biother Radiopharm 2000;15:339-46.
32. Sun SS, Hsieh JF, Tsai SC, Ho YJ, Kao CH. Technetium-99m tetrofosmin mammoscintigraphy findings related to the expression of P-glycoprotein mediated multidrug resistance. Anticancer Res 2000;20:1467-70.
33. Fuster D, Vinolas N, Mallafre C, Pavia J, Martin F, Pons F. Tetrofosmin as predictors of tumour response. Q J Nucl Med 2003;47:58-62.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Sioka C, Exarchopoulos T, Skloupiotis V, Papathanassiou V, Ragos V, Argyropoulou M, Gousia A, Tsekeris P, Exarchakos G, Assimakopoulos D, Fotopoulos A. The role of technetium Tc99m-tetrofosmin as head and neck tumor-seeking agent: a preliminary report. J Cancer Metastasis Treat 2016;2:133-8. http://dx.doi.org/10.20517/2394-4722.2015.65
AMA Style
Sioka C, Exarchopoulos T, Skloupiotis V, Papathanassiou V, Ragos V, Argyropoulou M, Gousia A, Tsekeris P, Exarchakos G, Assimakopoulos D, Fotopoulos A. The role of technetium Tc99m-tetrofosmin as head and neck tumor-seeking agent: a preliminary report. Journal of Cancer Metastasis and Treatment. 2016; 2: 133-8. http://dx.doi.org/10.20517/2394-4722.2015.65
Chicago/Turabian Style
Sioka, Chrissa, Thomas Exarchopoulos, Vlasis Skloupiotis, Vaios Papathanassiou, Vasileios Ragos, Maria Argyropoulou, Anna Gousia, Periklis Tsekeris, Georgios Exarchakos, Dimitrios Assimakopoulos, Andreas Fotopoulos. 2016. "The role of technetium Tc99m-tetrofosmin as head and neck tumor-seeking agent: a preliminary report" Journal of Cancer Metastasis and Treatment. 2: 133-8. http://dx.doi.org/10.20517/2394-4722.2015.65
ACS Style
Sioka, C.; Exarchopoulos T.; Skloupiotis V.; Papathanassiou V.; Ragos V.; Argyropoulou M.; Gousia A.; Tsekeris P.; Exarchakos G.; Assimakopoulos D.; Fotopoulos A. The role of technetium Tc99m-tetrofosmin as head and neck tumor-seeking agent: a preliminary report. J. Cancer. Metastasis. Treat. 2016, 2, 133-8. http://dx.doi.org/10.20517/2394-4722.2015.65
About This Article
Copyright
Data & Comments
Data

 Cite This Article 5 clicks
Cite This Article 5 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.