Percutaneous, computed tomography guided neurolysis using continuous radiofrequency for pain reduction in oncologic patients
Abstract
Aim: This study evaluates the efficacy and safety of percutaneous computed tomography (CT)-guided neurolysis using continuous radiofrequency for pain reduction in oncologic patients.
Methods: Over the course of 16 months, 22 patients underwent radiofrequency neurolysis as palliative therapy for pain reduction in celiac and splachnic plexus (n = 9), thoracic (n = 1), lumbar (n = 2) and superior hypogastric plexus (n = 5), as well as stellate ganglion (n = 5). Pain levels before treatment, one week after treatment, and at the last follow-up (average follow-up 6 months) were compared by means of a Numeric Visual Scale (NVS) questionnaire and a Brief Pain Inventory (Short Form) questionnaire.
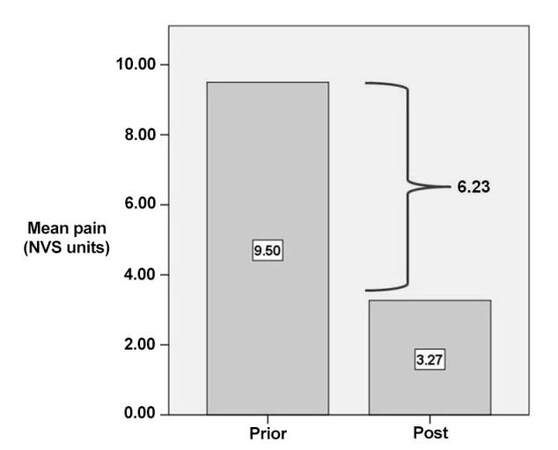
Results: Median procedure time was 44 min. Median number of CT scans, performed to control correct positioning of the cannula and precise electrode placement, was 8. Pain scores of questionnaires prior to treatment (mean value 9.50 NVS units, range 8-10 NVS units) and post treatment (mean value 3.27 NVS units, range 2-6 NVS units) showed a mean decrease of 6.23 NVS units in terms of pain reduction and life quality improvement (P < 0.05). Overall mobility improved in 18/18 (100%) patients. No complication was observed.
Conclusion: This study concludes that CT-guided neurolysis by means of continuous radiofrequency ablation is a safe and efficient technique for pain palliation in oncologic patients.
Keywords
Introduction
Approximately half of cancer patients report pain at the time of diagnosis, and nearly 80% of advanced stage cancer patients report moderate to severe pain.[1] Cancer pain can be classified as nociceptive (caused by ongoing tissue damage) or neuropathic (caused by damage or dysfunction in the nervous system), or mixed pain.[2,3] The World Health Organization (WHO) analgesic ladder was introduced in 1986 as a simplified model of analgesics escalation.[4] The three-step analgesic ladder proposed by WHO provides satisfactory pain management in a significant proportion of cancer patients. However, nearly one third of oncologic patients complain of refractory (nonresponsive) pain.[5] Despite its value, opioid administration can be costly; additionally, dose and continuous use relate directly to risk of harm.[6,7] Recently, a fourth step was proposed for refractory pain, which includes minimally invasive percutaneous techniques.
Radiofrequency ablation has been used since the 1970s for chronic pain therapy in cases of refractory pain. Ablation with continuous radiofrequency results in high temperatures (60-80 °C) that promote neurolysis and destroy the target nerves.[8,9] In contrast, application of pulsed radiofrequency maintains the temperature below 42 °C, promoting neuromodulation of the target nerves.[10,11] Both continuous and pulsed radiofrequency modes have been applied for pain reduction in symptomatic cancer patients with refractory pain. Percutaneous neurolysis can be either chemical (phenol or alcohol injection) or thermal (radiofrequency or cryoablation). During continuous radiofrequency (RF) ablation an electrode is placed close to a nerve fiber, applying energy which is transformed to high temperature causing protein denaturation and destruction of the axons, resulting in transmission blockage of nociceptive signals from the periphery.[12]
The purpose of this study is to evaluate efficacy and safety of percutaneous computed tomography (CT)-guided neurolysis using continuous radiofrequency for pain reduction in oncologic patients with pain refractory to standard treatments proposed in the WHO three-step analgesic ladder.
Methods
All patients were informed about the technique itself as well as about possible benefits and complications. All patients signed a written consent form for the procedure. Authors have no conflict of interest to declare. No industry support was received for this study.
Patient selection
This is a retrospective study evaluating a consecutive series of patients undergoing CT-guided neurolysis using continuous radiofrequency ablation. During the last 16 months, 22 patients (10 males/12 females) suffering from cancer pain refractory to systemic therapy with opioids and adjuvant drugs were referred for percutaneous CT-guided neurolysis as palliative therapy for pain reduction. All patients treated had no contraindications for regional blockade. Malignant background included pancreatic carcinoma (n = 8), pancoast tumor (n = 5), lymphoma (n = 1), renal cell (n = 1), endometrial (n = 2), colon (n = 2) and ovarian (n = 3) carcinoma. Patients underwent neurolysis by means of radiofrequency (Diros Technology In, Ontario Canada) in celiac and splanchnic plexus (n = 9), in thoracic (n = 1), in lumbar (n = 2), in superior hypogastric plexus (n = 5) and in stellate ganglion (n = 5) [Figure 1].
Technique
All procedures were performed in the CT room, under anesthetic monitoring and strict aseptic technique by two interventional radiologists with cooperation of an anesthesiologist. Unilateral or bilateral approach was used, depending on the blockage that was performed. The procedural route and site of the skin puncture were determined with CT guidance. Once the puncture site was located, local anesthetic (5-10 mL of Lidocaine Hydrochloric 2%) was injected into the subcutaneous soft tissue. Under continuous CT scans at the level of interest, 20 G trocar(s) was (were) percutaneously inserted and advanced. The final trocar position was verified with CT scan post contrast medium injection.
For the celiac plexus neurolysis, a posterior transcrural approach was used, with needles passing through the diaphragmatic crura in route to the celiac plexus anterolateral to the aorta. For the neurolysis of the splanchnic nerves, a retrocrural approach was performed with the needles remaining posterior to the diaphragmatic crura and placed at the level of L1 vertebral body (cephalad half) and at midportion of T12 vertebral body. For the lumbar plexus neurolysis, two or three needles were placed over the transverse process of L2, L3 and L4 vertebrae, respectively, with the needle tip at the anteromedial vertebral body surface where the lumbar sympathetic block lies. For the superior hypogastric neurolysis, the needle was placed at the anterolateral surface of L5-S1 intervertebral disc, either via posterolateral access through the sacral ala and the superior articular process of S1 or via transdiscal access. Thoracic neurolysis was performed with the needles placed at the space between vertebral body (lateral aspect) and pleura at T2 and T3 levels.
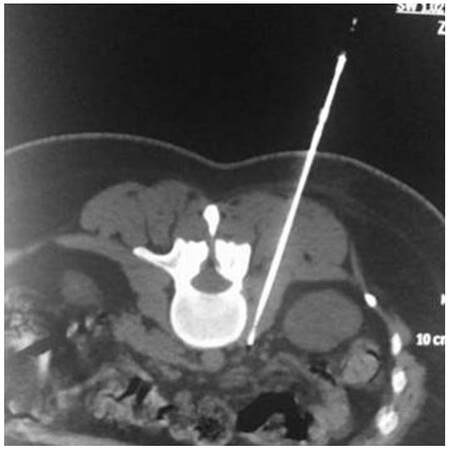
Coaxially, the RF electrode was then inserted [Figures 2-4]. Motor and sensory tests were performed to verify the electrode’s correct position near the sensory nerve segment and away from the motor root. Upon satisfactory test results, two CRF ablation sessions were performed at 80 °C, with total duration time of 90 s each. All patients were closely observed postoperatively for pain, sensory and motor deficits, as well as for vital signs. Patients remained in the hospital overnight for hydration and observation and exited the morning after the procedure.
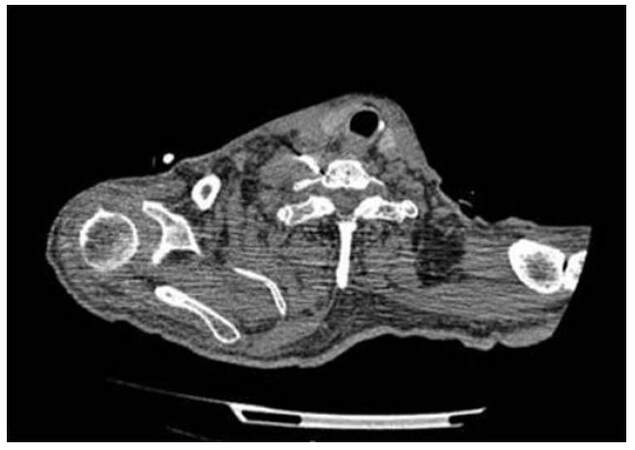
Figure 2. Stellate ganglion neurolysis: the ganglion is usually located lateral to the outer border of longus colli muscle anterior to the neck of the first rib and the transverse process of the 7th cervical vertebra
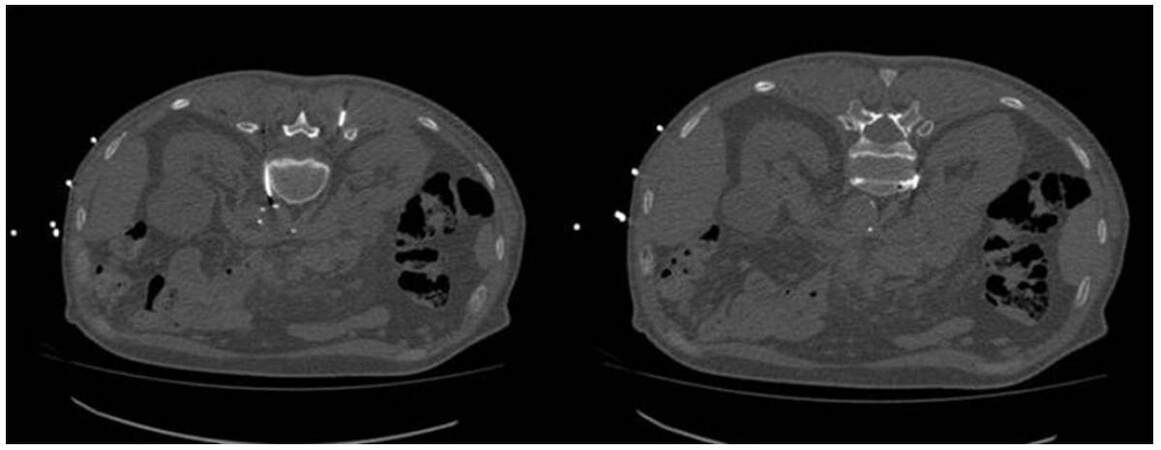
Figure 3. Splachnic neurolysis is a modification of the classic retrocrural approach for celiac plexus. Needles are placed at the midportion of T12 vertebral body
Outcome measures
Pain assessment was performed using the Numeric Rating Scale (NRS, 0-10) questionnaire and Brief Pain Inventory (Short Form) questionnaire for reviewing quality of life.[19] The questionnaires were recorded before the treatment, one week after treatment, and at the last follow-up (average follow-up 6 months).
Results
Twenty-two patients were studied, all suffering from cancer pain refractory to systemic therapy with opioids and adjuvant drugs. All patients completed the follow-up of six months.
Median procedure time was 44 min. Median number of CT scans, performed to control correct positioning of the cannula and precise electrode placement, was 8. No complications occurred during the procedure, and all patients tolerated the procedure well.
Comparing the self-reported pain scores of questionnaires prior to treatment (mean value 9.50 NRS units, range 8-10 NRS units) and at 6 months post treatment (mean value 3.27 NRS units range 2-6 NRS units), there was a mean decrease of 6.23 NRS units in terms of pain reduction and life quality improvement [Figure 5]. Overall mobility improved in 18/18 (100%) patients.
Discussion
Cancer pain has direct implications for patients’ quality of life. Cancer pain can be classified as nociceptive (described as somatic or visceral and caused by ongoing tissue damage) or neuropathic (caused by damage or dysfunction in the nervous system).[2,3] The WHO analgesic ladder has three steps for acute pain, chronic pain without control, or acute crises of chronic pain. In step 1, nonopioids, analgesics, and NSAIDs are administered to the patient. In step 2, weak opioids can be added to the treatment regime. In step 3, methadone or strong opioids can be administered orally or by means of a transdermal patch.
Unfortunately, conservative therapy does not adequately reduce pain in the vast majority of oncologic patients (56% to 82.3%).[2,3] On the other hand, numerous studies in the literature report significant pain reduction post chemical or thermal neurolysis.[13-16] Papadopoulos et al.,[17] who conducted treatment with radiofrequency ablation of splanchnic nerves on 35 patients with end-stage pancreatic abdominal cancer pain refractory to conservative treatment, reported significant decrease in pain scores and consumption of opioids and significant improvement in the patient quality of life during a follow-up period of 6 months.
Our study included patients with a diversity of malignant substrate, evaluating the efficacy of RF neurolysis in celiac and splanchnic plexus (n = 9), in the thorax (n = 1), in the lumbar region (n = 2), in superior hypogastric plexus (n = 5), and in the stellate ganglion (n = 5). The results of our study (statistically significant mean decrease of 6.23 NVS units on terms of pain reduction and life quality improvement) are in agreement with the previously mentioned success rates.
Percutaneous neurolysis has been reported as a safe procedure with a low complications rate. The most commonly reported complications include transient diarrhea (10-25%), orthostatic hypotension (20-42%), and local pain. Rarer complications include paresis, pneumothorax, shoulder pain (1%), hemorrhagic gastritis, duodenitis, and death.[18-20] In our study we performed continuous RF neurolysis in all our patients, and we did not experience any complications. We believe that continuous RF neurolysis has a shorter risk-benefit ratio than alcohol neurolysis, since it is a more sophisticated and targeted interventional technique. When compared to medical management by opioids, percutaneous neurolysisis superior in terms of fewer burdensome side effects.[5,21,22]
Correct cannula positioning should always be verified with electrical stimulation prior to ablation. Two stimulation types are performed: sensory and motor. Successful electrical sensory stimulation triggers pain that aligns with the patient’s usual distribution of pain. When motor stimulus is performed, there should be no motor response in a threshold below 2.0 volts or below twice the threshold value of the sensory test. Sensory testing increases the technique’s efficacy, and motor stimulation ensures safety from motor impairment.
Limitations of our study include its retrospective nature as well as the diversity of the malignant substrate. Additionally, there was no randomized comparison between continuous RF neurolysis and placebo therapy, chemical neurolysis with phenol or alcohol, or medical management.
Radiofrequency neurolysis under CT guidance is feasible and reproducible, efficient (70-80% success rate), and safe (> 0.5% mean complications rate) as palliative therapy for pain reduction in oncologic patients with refractory pain. Thorough knowledge of nervous system anatomy and pain transmission pathways is essential for proper patient and technique selection.
Authors’ contributions
Text writing: P. Zavridis, A. Mazioti
Statistic analysis: M. Tsitskari
Submission editing: D. Filippiadis
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interests.
Patient consent
Patient consent was obtained.
Ethics approval
Ethics approval was obtained.
REFERENCES
2. Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J; ESMO Guidelines Working Group. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2014;25 Suppl 3:iii124-37.
3. Ripamonti CI, Santini D, Maranzano E, Berti M, Roila F; ESMO Guidelines Working Group. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol 2012;23 Suppl 7:vii139-54.
4. World Health Organization. Cancer pain relief: with a guide to opioid availability. 2nd ed. 1996. Available from: http://apps.who.int/iris/bitstream/10665/37896/1/9241544821.pdf.
5. Vayne-Bossert P, Afsharimani B, Good P, Gray P, Hardy J. Interventional options for the management of refractory cancer pain--what is the evidence? Support Care Cancer 2016;24:1429-38.
6. Martin BC, Fan MY, Edlund MJ, Devries A, Braden JB, Sullivan MD. Long-term chronic opioid therapy discontinuation rates from the TROUP study. J Gen Intern Med 2011;26:1450-7.
7. Ballantyne JC, Kalso E, Stannard C. WHO analgesic ladder: a good concept gone astray. BMJ 2016;352:i20.
8. Vallejo R, Benjamin RM, Aliaga L. Radiofrequency vs. pulse radiofrequency: the end of the controversy. Tech Region Anesth Pain Manage 2010;14:128-32.
9. de Louw AJ, Vles HS, Freling G, Herpers MJ, Arends JW, Kleef M. The morphological effects of a radio frequency lesion adjacent to the dorsal root ganglion (RF-DRG) -- an experimental study in the goat. Eur J Pain 2001;5:169-74.
10. Kvarstein G. Pulsed radiofrequency -- time for a clinical pause and more science. Scand J Pain 2012;3:124-6.
11. Cahara A, van Zundert J, Macrea L, van Kleef M, Sluijter M. Pulsed radiofrequency: current clinical and biological literature available. Pain Med 2006;7:411-23.
12. Malik K, Benzon HT, Walega D. Water-cooled radiofrequency: a neuroablative or a neuromodulatory modality with broader applications? Case Rep Anesthesiol 2011;2011:263101.
13. Locklin JK, Mannes A, Berger A, Wood BJ. Palliation of soft tissue cancer pain with radiofrequency ablation. J Support Oncol 2004;2:439-45.
14. Bhaskar AK. Interventional management of cancer pain. Curr Opin Support Palliat Care 2012;6:1-9.
15. de Courcy JG. Interventional techniques for cancer pain management. Clin Oncol (R Coll Radiol) 2011;23:407-17.
16. Uchida K. Radiofrequency treatment of the thoracic paravertebral nerve combined with glucocorticoid for refractory neuropathic pain following breast cancer surgery. Pain Physician 2009;12:E277-83.
17. Papadopoulos D, Kostopanagiotou G, Batistaki C. Bilateral thoracic splanchnic nerve radiofrequency thermocoagulation for the management of end-stage pancreatic abdominal cancer pain. Pain Physician 2013;16:125-33.
18. Vissers KC, Besse K, Wagemans M, Zuurmond W, Giezeman MJ, Lataster A, Mekhail N, Burton AW, van Kleef M, Huygen F. 23. Pain in patients with cancer. Pain Pract 2011;11:453-75.
19. Zhong W, Yu Z, Zeng JX, Lin Y, Yu T, Min XH, Yuan YH, Chen QK. Celiac plexus block for treatment of pain associated with pancreatic cancer: a meta-analysis. Pain Pract 2014;14:43-51.
20. Plancarte R, de Leon-Casasola OA, El-Helaly M, Allende S, Lema MJ. Neurolytic superior hypogastric plexus block for chronic pelvic pain associated with cancer. Reg Anesth 1997;22:562-8.
21. Arcidiacono PG, Calori G, Carrara S, McNicol ED, Testoni PA. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev 2011;(3):CD007519.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Zavridis P, Tsitskari M, Mazioti A, Filippiadis D. Percutaneous, computed tomography guided neurolysis using continuous radiofrequency for pain reduction in oncologic patients. J Cancer Metastasis Treat 2017;3:60-4. http://dx.doi.org/10.20517/2394-4722.2017.04
AMA Style
Zavridis P, Tsitskari M, Mazioti A, Filippiadis D. Percutaneous, computed tomography guided neurolysis using continuous radiofrequency for pain reduction in oncologic patients. Journal of Cancer Metastasis and Treatment. 2017; 3: 60-4. http://dx.doi.org/10.20517/2394-4722.2017.04
Chicago/Turabian Style
Zavridis, Periklis, Maria Tsitskari, Argyro Mazioti, Dimitrios Filippiadis. 2017. "Percutaneous, computed tomography guided neurolysis using continuous radiofrequency for pain reduction in oncologic patients" Journal of Cancer Metastasis and Treatment. 3: 60-4. http://dx.doi.org/10.20517/2394-4722.2017.04
ACS Style
Zavridis, P.; Tsitskari M.; Mazioti A.; Filippiadis D. Percutaneous, computed tomography guided neurolysis using continuous radiofrequency for pain reduction in oncologic patients. J. Cancer. Metastasis. Treat. 2017, 3, 60-4. http://dx.doi.org/10.20517/2394-4722.2017.04
About This Article
Copyright
Data & Comments
Data
 Cite This Article 5 clicks
Cite This Article 5 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.