Significance of peritoneal lavage cytology based on genetic signatures in gastric cancer
Abstract
Peritoneal metastasis is the most common pattern of recurrence and the most frequent cause of death after surgery in patients with gastric cancer. Peritoneal free cancer cells disseminated from the primary lesion site have been considered the main cause of peritoneal metastasis. Peritoneal lavage cytological examination (PLC) has been shown to be an independent predictor of gastric cancer relapse after curative resection and poor overall survival. However, the conventional cytological examinations have high rates of false-positive and false-negative findings. To improve the sensitivity, molecular-based methods using reverse transcriptase polymerase chain reaction have been developed for detecting cancer cells in peritoneal wash fluids of patients with gastric cancer. We performed a PubMed search for articles describing PLC in gastric cancer. Relevant articles were reviewed and data on available outcomes elaborated. The clinical roles and attributes of PLC in gastric cancer were reviewed, and its future application to this disease is discussed.
Keywords
Introduction
Gastric cancer is the most common malignancy worldwide and the second leading cause of cancer-related death[1]. Despite the development of surgical techniques and new therapeutic strategies, the outcome of patients with advanced gastric cancer is still unsatisfactory[2]. Peritoneal dissemination is the most common pattern of metastasis or recurrence, and is the most frequent cause of death after surgery in patients with gastric cancer. Intraperitoneal free cancer cells exfoliated from the cancer-invaded serosa has been considered the main cause of peritoneal dissemination[3]. Therefore, cytological examination of peritoneal lavage fluid (PLF) obtained at the time of surgery has been considered a useful tool to detect free cancer cells. The peritoneal lavage cytological examination (PLC) has been regarded as a feasible and indeed, the most effective method to predict peritoneal recurrence and survival in patients with gastric cancer[4-6].The Japanese Gastric Cancer Association suggests that the presence of free cancer cells in the peritoneal cavity should be considered an independent prognostic factor in patients with gastric cancer[7]. In addition, positive PLC is defined as distant metastasis in the seventh edition of the American Joint Committee on Cancer Staging[8]. Therefore, patients with positive PLC most likely will not derive a benefit from surgical procedure, and should be offered systemic chemotherapy or palliative therapy. Recent progress in systematic chemotherapy has resulted in the improvement of prognosis and has allowed the introduction of conversion surgery for select patients who respond effectively to the chemotherapy. However, there are still many issues to address, as critical evidence regarding the timing of conversion, optimal chemotherapy regimen(s), and period of chemotherapy does not exist at present.
Conventional cytology to detect cancer cells in PLF has been performed routinely[4]. However, the fact that peritoneal recurrence can be detected in approximately 10% of patients with negative PLF cytology suggests that this cytological examination might not be sufficient for the detection of free cancer cells and prediction of peritoneal spread[9]. A more sensitive method for detecting free cancer cells in the peritoneal cavity is urgently needed. The ability to predict micrometastasis development would significantly advance the therapeutic approach to gastric cancer. Over the last few decades, many investigators have proposed the use of molecular diagnostic methods, such as reverse transcription-polymerase chain reaction (RT-PCR) targeting various clinical fields, including detection of free cancer cells[3,10,11]. Hence, in an effort to achieve early detection, the analysis of a patient’s “genetic signature” using PLF after curative surgery has been employed in recent years, especially in gastric cancer. In this review, we discuss the current evidence and future perspectives of PLC for gastric cancer.
Methods
PubMed was searched for English articles using the medical subject headings “gastric cancer”, “peritoneal lavage”, and “peritoneal washing”. Relevant articles from clinical trials and case reports since 1989 were included, as well as background articles relevant to the disease processes of interest.
Background of PLC
To detect free cancer cells and to predict peritoneal metastasis, conventional PLC performed on PLF obtained during surgery has been broadly used[4,12]. PLC is currently examined via Papanicolaou staining and assessed by a cytopathologist. Positive free cancer cells in PLC have been shown to be an important and independent prognostic factor in patients with gastric cancer[12]. Thus, PLC has been recommended in the Japanese Classification of Gastric Carcinoma from 1998 onward[13]. However, conventional PLC is positive in only 59% of patients with macroscopical peritoneal disease[14]. Additionally, the conventional cytology in patients without any macroscopic peritoneal metastasis after curative surgery shows much lower sensitivity (5%-15%)[15,16]. Meanwhile, levels of traditional tumor markers associated with gastric cancer in PLF have been calculated to obtain greater sensitivity. Carcinoembryonic antigen (CEA) is a glycoprotein found in colon cancer; it plays a role in cell adhesion[17]. Although CEA is not sufficient with regard to diagnostic sensitivity and specificity for early gastric cancer, guidelines suggest that serum CEA levels should be measured to predict recurrent gastric cancer[18]. It has been reported that CEA levels in PLF accurately predict peritoneal recurrence after a curative resection of gastric cancer[19]. The addition of immunohistochemical CEA measurement to conventional cytology resulted in increased sensitivity (26%)[20]. Combined analysis of CEA with other principal gastric tumor markers, such as CA72-4 or CA125, has been shown to enhance the accuracy for predicting peritoneal relapse[21,22]. Regardless, the CEA measurement in PLF still has an about 20% false negative for peritoneal dissemination[9]. Thus, there is still a need for more sensitive methods of PLC with lower false-positive and false-negative rates.
Genetic detection of PLC
The greater sensitivity of RT-PCR analysis has made it possible to detect micrometastasis in the basis of cancer-tissue-specific messenger RNA (mRNA) expression in peripheral veins, lymph nodes, bone marrow, and the peritoneal cavity[23]. Molecular diagnosis using RT-PCR is generally reflected to be a more sensitive and quantitative method than conventional cytology for the detection of micrometastasis in PLC[3,10]. Thus, RT-PCR analysis of PLF should have clinical significance in the diagnostic evaluation of suspected peritoneal metastasis and the development of therapeutic strategies. Based on a range of studies, there is a robust correlation between the results of RT-PCR analysis of PLF and prognosis after curative operation in patients with advanced gastric cancer [Table 1]. In this section, we will discuss selected molecular markers of PLC including those based on genetic approaches.
List of published studies regarding the genetic diagnosis of peritoneal lavage cytology in gastric cancer
| Study | Year | Marker | Detection method | Number of patients | Main results |
|---|---|---|---|---|---|
| Nakanishi et al.[24] | 1997 | CEA | RT-PCR | 48 | RT-PCR is more sensitive for detection of free carcinoma cells in the peritoneal cavity than conventional cytology |
| Fujimura et al.[53] | 1998 | Trypsinogen | RT-PCR | 30 | Trypsinogen-1 mRNA was positive for the patient, who did not show macroscopic or cytological peritoneal dissemination |
| Yonemura et al.[25] | 2001 | MMP-7 | RT-PCR | 152 | Improved the sensitivity for peritoneal dissemination in combination with cytology |
| Kodera et al.[34] | 2002 | CEA | RT-PCR | 90 | PCR positive was a significant independent prognostic factor, but CY positive was not |
| Sugita et al.[39] | 2003 | CEA, CK20 | RT-PCR | 129 | In cases with negative cytology, patients with PCR-positive findings in PLF had a poorer outcome than those with negative PCR |
| Mori et al.[66] | 2004 | Multiple marker | Microarray | 179 | Correlation with disease-free survival and immunocytochemical cytology |
| Wang et al.[15] | 2005 | CEA | RT-PCR | 40 | The technique of RT-PCR was more sensitive than conventional PLC in the detection of peritoneal free cancercells and the prediction of peritoneal recurrence |
| Kodera et al.[38] | 2005 | CK20 | RT-PCR | 195 | Not sufficiently sensitive compared with CEA |
| Ohashi et al.[35] | 2007 | CEA | TRC method | 112 | TRC has a diagnostic power almost equivalent to qRT-PCR but with the advantage of ultra-rapid detection |
| Da et al.[57] | 2007 | Telomerase activity | TRAP assay | 60 | Correlation with high proliferating activity of gastric cancer |
| Hiraki et al.[45] | 2011 | Aberrant gene methylation | Metylation-specific PCR | 107 | Methylation analysis along with a cytological examination might therefore improve the positive detection of cancer cells in PF of gastric cancer |
| Horikawa et al.[62] | 2011 | CD44, CD45, EpCAM | RT-PCR | 147 | CD44 mRNA of magnetically separated CD45EpCAM+ cell fraction of PLC is useful for predicting high-risk individuals among gastric cancer patients with stage II and III |
| Takata et al.[41] | 2014 | CEA, CK20 | RT-PCR | 104 | CEA and CK20 PCR results could predict peritoneal recurrence after curative surgery |
| Li et al.[51] | 2014 | CEA, MMP-7 | RT-PCR | 116 | CEA and MMP-7 transcripts in PLF could effectively predict peritoneal recurrence |
| Jeon et al.[43] | 2014 | CEA, MAGE | RT-PCR | 117 | MAGE expression was determined to be the most important prognostic factor for recurrence |
| Tokuhisa et al.[47] | 2015 | Exosomal miRNAs | Agilent Human miRNA microarrays and qRT-PCR | 24 | miRNA expression profiles can indicate the status of peritoneum in GC patients |
| Miwa et al.[49] | 2017 | FBXO50 | The ABI StepOnePlus Real-Time PCR System and TaqMan Copy Number Assay | 200 | FBXO50 expression related with recurrence after curative gastrectomy and shorter overall survival |
Carcinoembryonic antigen
Studies conducted over the last couple of decades have demonstrated the usefulness of measuring CEA mRNA to detect micrometastasis in the peritoneal cavity. Nakanishi et al.[24] were the first to describe the high sensitivity for detecting free cancer cells through RT-PCR amplification of CEA mRNA. Positive rate of analysis through RT-PCR was elevated to 20% than that of cytology alone[24]. Subsequent to their study, many further studies examining CEA mRNA in PLF as the target molecular marker in gastric cancer were published[11,15,25-32]. For instance, one study using RT-PCR for CEA mRNA showed a detection rate of free cancer cells of 28%, with a 14% higher detection rate than for PLC[33]. A recent prospective study of quantitative CEA mRNA detection in PLF using the most desirable cutoff value of CEA mRNA of 0.1 by ROC curve analyses found that the positive rates for CEA mRNA were 45.7% and 50.0% in T3 and T4 patients, respectively. Among the CEA mRNA-positive patients, 55.0% induced peritoneal metastasis. In contrast, only 3.0% of patients who were negative for CEA mRNA had peritoneal relapse, 84.6% of the positive rate of CEA mRNA in PLF from patients with peritoneal dissemination for the period of postoperative surveillance. CEA mRNA was shown to be only an independent prognostic factor in multivariate analysis with peritoneal recurrence-free survival[30].
Nevertheless, the sensitivity of the CEA RT-PCR assay for detecting peritoneal micrometastasis is still insufficient. Moreover, false positive results, caused by expression of CEA in no malignant cells such as mesothelial cells and lymphocytes, remains a key problem of this technique[34]. To overcome these problems, a recent study showed that a novel and rapid molecular method of diagnosis using the technique of transcription-reverse transcriptase concerted reaction (TRC) has been developed[35,36]. A prospective study at multiple institutions to examine the clinical benefit of TRC diagnosis with PLF from gastric cancer patients was carried out. Accordingly, TRC can be a prognostic factor for the prediction of patient outcome and peritoneal metastasis of gastric cancer with serosa-infiltrating tumors. On the other hand, another paper showed that CEA mRNA index (CmRI) (CEA mRNA/porphobilinogen deaminase mRNA × 10,000) values in PLF may be a useful tool for reflecting the response of peritoneal relapse to induction chemotherapy and that the advantage of conversion gastric surgery could be predicted by CmRI values[37].
Cytokeratin
Keratins are intermediate filament proteins which are closely related with the structural integrity of epithelial cells. Recently, several studies have identified cytokeratin-20 (CK-20) as one of the potential cancer-related biomarkers for the detection of peritoneal free cancer cells for the patients with gastric cancer[38,39]. CK-20 has been used as a factor with CEA in a multiple-marker analysis for the detection of peritoneal micrometastasis[40]. In a recent study, real-time quantitative RT-PCR analysis of the CEA and/or CK20 transcripts in PLF was presented to be useful for predicting peritoneal metastasis in patients after curative resection for gastric tumor. The sensitivities of combined CEA and/or CK20 mRNA levels were 86.4% and 81.5%, respectively, clearly increased compared with that of each marker alone. In the patients with a curative resection, the survival rate of the PCR-positive was significantly lower than that of PCR-negative in the gastric cancer patients with a curative surgery. Additionally, the level of CEA or CK20 mRNA was an independent prognostic factor for overall survival rate[40]. Another prospective study also found that CEA and CK20 RT-PCR results could predict peritoneal recurrence after curative surgery[41].
Melanoma associated gene
Melanoma associated gene (MAGE) has been said to be a cancer-specific marker responsible for the suppression of apoptosis and carcinogenesis[42]. RT-PCR of gastric cancer shows that the MAGE genes are highly expressed than that of other markers[43]. Although the rate of expression differs in accordance with subtype, expressions of at least one of MAGE-4, MAGE-6, MAGE-8, MAGE-9, MAGE-10, and MAGE-12 genes were as high as 82% in gastric cancerous specimen[44]. Furthermore, previous studies reported that MAGE was not expressed in normal gastric tissue[44]. These results suggest that MAGE has been a candidate as a novel targeted gene for the prediction of survival in patients with gastric cancer, and is expected to be a therapeutic target due to its specific expression. A recent report in trial comparing the two markers CEA and MAGE demonstrated that superior specificity and important association with peritoneal metastasis were revealed in MAGE RT-PCR than in CEA RT-PCR after long-term follow-up, and MAGE RT-PCR results were shown to be the most significant survival factor for peritoneal relapse in patients with gastric cancer after curative surgical procedure[43].
Gene methylation
To identify micrometastasis in salivary rinses for head-and-neck cancer patients and pleural effusion for several cancers, cancer-specific gene methylation has been commonly investigated. Thus, aberrant gene methylation in PLF may predict peritoneal recurrence in gastric cancer. A previous study evaluated whether methylation in the PLF by quantitative methylation-specific PCR analysis affects peritoneal metastasis after surgery in the patients in which the depth of invasion of the primary lesion was beyond the muscularis propria[45]. Twelve-fold enhanced risk of peritoneal relapse in patients with positive methylation was shown compared with in those with negative methylation by the combined assessment of the 6 genes (BNIP3, CHFR, CYP1B1, MINT25, RASSF2, and SFRP2). Additionally, positive methylation rate in patients with peritoneal metastasis or positive PLC was increased up to 75% by the combined assessment of the 6 genes, whereas the rate in gastric cancer patients with the depth of cancer invasion beyond the muscularis propria (that is, tumor involves the subserosa, tumor penetrates the serosa, and tumor invasion of adjacent structures present) was 20%.
Exosomal miRNAs
MicroRNAs (miRNAs) are small non-coding RNAs that serve as posttranscriptional regulators of gene expression and have an essential role in the control of many biological processes[46]. A recent study investigated the diagnostic potential of exosomal miRNA profiles in peritoneal fluid for the prediction of peritoneal dissemination in gastric cancer[47]. The miRNA content of exosomes isolated from malignant ascites and peritoneal lavage fluid of gastric cancer patients was examined by miRNA microarray technology. Significant high expressions of miR-21 and miR-1225-5p were found in patients with T4-stage cancer than that in T1- to T3-stage patients, suggesting that profiling of miRNAs in peritoneal lavage fluid may be used for the prediction of a peritoneal premetastatic phenotype in gastric cancer and may provide more effective preventive and curative measures.
FBXO50
F-box proteins, which are the substrate-recognition subunits of SKP1-cullin 1-F-box protein E3 ligase complexes, play essential roles in a variety of cellular processes through ubiquitylation which lead to the degradation of target proteins[48]. F-box only proteins (FBXOs) are key subclass of F-box proteins organized in accordance with the existence of specific substrate recognition domains. Expression levels of FBXO50 mRNA in gastric cancer tissues from 200 patients were investigated, and the level of FBXO50 expression was significantly correlated with positive peritoneal lavage cytology[49]. FBXO50 would be another new candidate tool of PLC for detecting micrometastasis in gastric cancer.
Other genetic markers
Besides the markers described above, numerous different markers to detect micrometastasis including various aspects of biological activity in gastric cancer are known. The genetic alteration of proteinases, which are closely associated with cancer invasion, has been regarded as one of the useful tools in the early detection of peritoneal metastasis. Matrix metalloproteinase 7 (MMP-7), also called matrilysin, is a familiar member in the MMP family due to its excessive proteolytic activity for a broad range of molecules and is selectively produced from gastric cancer cells[50]. Moreover, a previous study found that an MMP-7 RT-PCR assay of PLF detected cancer cells at densities of as low as < 10 cells/sample and was an independent predictor of peritoneal recurrence[25]. A quantitative RT-PCR analysis of the CEA and MMP-7 transcripts in the PLF effectively predicted peritoneal relapse in gastric cancer in multivariate analysis, and combination analysis of them enhanced the sensitivity and specificity compared with conventional PLC (71.1% and 74.6%, respectively)[51]. Trypsin is a member of the serine protease family which consists of 3 trypsinogen genes (trypsinogen 1, 2 and 4) and has a potential role in cancer invasion[52]. As a major digestive enzyme, trypsin has high proteolytic activity, and its unsuitable activation may result in peritoneal dissemination of infiltrative gastric cancer. Trypsinogen may be a good candidate for the early detection of peritoneal recurrence in gastric cancer, because trypsinogen-1 mRNA was positive in a patient who did not show macroscopic or cytological peritoneal dissemination[53]. Th17 cells have been identified as having a distinct Th cell lineage and have been found in several types of human cancers, including gastric cancer[54]. Increasing evidence suggests that IL-17 promotes tumor growth through angiogenesis and inflammation. On the other hand, it contributes to the reduction of tumor growth by promoting dendritic cells, cytotoxic T lymphocyte, and NK cells. Patients with high expression of IL-17 mRNA detected by real-time RT-PCR in peritoneal lavage showed significantly prolonged survival compared with patients with low expression of IL-17 mRNA in peritoneal lavage, suggesting that low IL-17 gene expression in PLF may correlate with cancer development and poor prognosis in patients with gastric cancer[55]. Telomerase is a ribonucleoprotein polymerase that adds TTAGGG repeats to telomeric ends. Telomerase regulates cellular immortality and is reactivated in approximately 85% of human malignancies[56]. A recent study using a telomeric repeated amplification protocol - enzyme-linked immunosorbent assay found that telomerase activity in PLF can be detected in patients with peritoneal metastasis, and found the positive rate of telomerase activity was significantly associated with the positive rate of telomerase activity and the presence of peritoneal recurrence, although these methods were not superior to conventional cytology by itself[57].
Other candidates for genetic marker in PLC
The approaches mentioned above focus on the detection of already known genetic changes, whereas full genome sequencing can be used for the detection of new candidates, and expression profiling may provide the detection of previously unknown markers for PLC. With regard to peritoneal metastasis, malignant features of tumor cells such as altered expression of growth factors, immuno-insufficiency, decreased intercellular adhesion, increased cell-to-matrix adhesion, and resistance to apoptosis are considered to be pivotal characteristics. The results of this comprehensive gene expression analysis of gastric cancer with peritoneal metastasis may provide new insight into the detection of micrometastasis in PLF. A previous study using a global analysis of the differential gene expression showed the relative mRNA levels of genes expressed in gastric cancer cell lines established from primary tumors and of other cell lines established from metastasis to the peritoneal cavity[58]. Twenty-four genes including CD44, dopa decarboxylase (DDC), keratin family genes, aldehyde dehydrogenase, CD9 and IP3 receptor type 3 were up-regulated while 17 genes including CD4, IL4 Stat, IGFBP2, and histon deacetylase 3 were down-regulated in the metastatic cell lines based on results of a high-density cDNA microarray method[58]. Among them, the precise roles of DCC in peritoneal metastasis have been investigated. DDC is an enzyme for the metabolism of dopamine, and is also responsible for the production of neurotransmitters, such as serotonin[59]. DCC was one of these upregulated genes. DDC-specific RT-PCR may become a novel marker for peritoneal dissemination of gastric cancer[60]. CD44-positive gastric cancer cells have been said to show properties of self-renewal and the capability to generate differentiated progeny, in line with the CSC[61]. CD44 mRNA of separated CD45 EpCAM-positive cell fraction of peritoneal washes using the Auto-MACS system may be a useful genetic marker for predicting high-risk individuals among stage II and III gastric cancer patients[62]. Phenotype L3-phosphoserine phosphatase (L3-PP) is also one of the highly-expressed genes that have been analyzed by high-density microarray. L3-PP encodes the phosphatase of phosphoserine and has been said to be involved in amino acid synthesis[63]. The enhancement of the activity of L3-PP has been found according to the increased cell multiplication and frequency of mitosis[63]. It has been reported that L3-PP overexpression of L3-PP in gastric cancer cells obtained from peritoneal metastasis by RT-PCR has been shown to be closely associated with peritoneal recurrence of gastric cancers[64]. Combined RT-PCR analysis of CEA with L3-PP resulted in the reduction of false negative CEA mRNA and increased sensitivity of peritoneal metastasis detection from 71.4% to 85.7%. Another study performed global analysis on differential gene expression of a scirrhous gastric cancer cell line (OCUM-2M) and its derivative sublines with high potential for metastasis to the peritoneal cavity (OCUM-2MD3) in a nude mouse model[65]. Twelve genes including rab32, trefoll factor 1, α-1-antitrypsin, and gelactin4, were up-regulated by applying a high-density oligonucleotide array method. Besides, RT-PCR was performed in 16 representative PLF samples to classify genes specific to cytology-positive samples[66]. The usefulness as markers for minimal resonant disease in 99 PLF sample was examined using 5 genes finally selected-CK20, FABP1, MUC2, TFF1, and TFF2. Positive findings which were highly specific to fatal cases (91%-100%) were found by nested RT-PCR using the 5 genes. With high specificity, the combined use of these 5 genes resulted in identifying 6 out of 20 (30%) additional patients with all kinds of early relapse.
Consequently, these genomic profiling findings suggest the critical importance of setting up a basis upon which to establish not only improved molecular understanding, but also better targeted strategies for gastric cancer treatment.
Clinical application
Up to the present, effective therapies for peritoneal metastasis have not been established. A previous study found that a new oral fluorinated pyrimidine agent (S-1), used as a postoperative monotherapy, did not show superior effect in survival in patients with macroscopic peritoneal tumor compared with patients with positive cytology[67]. To improve survival, it is essential to identify high-risk patients at an earlier phase of peritoneal metastasis. Several experimental studies have shown that micrometastases are more responsive to chemotherapy than visible metastatic tumors[68,69]. Thus, in addition to make an accurate diagnosis, molecular diagnosis using RT-PCR analysis has an important role in starting chemotherapy before the development of macrometastasis. A phase II clinical trial for evaluating the prognostic impact of postoperative S-1 monotherapy in gastric cancer patients with CEA mRNA positivity was carried out. Accordingly, the 3-year survival did not show the significant difference between the study population and the historic control (67.3% vs. 67.1%, respectively), suggesting that S-1 may delay cancer relapse but not always eradicate micrometastases[70].
Because micrometastases are more susceptible to chemotherapy than macroscopic disease, neo-adjuvant chemotherapy would theoretically has a benefit in this subgroup of patients, because micrometastases are more susceptible to chemotherapy than macroscopic disease[71]. Positive cytology may serve as a guide to continuing chemotherapy or changing the mode of therapy. Preoperative chemotherapy protocols may select patients more likely to benefit from resection. A previous study analyzed the genetic diagnosis using PLF for detecting patients at high risk for peritoneal recurrence and for evaluating the clinical response to intraperitoneal chemotherapy in patients with gastric cancer[66]. From nineteen patients with advanced gastric cancer who underwent staging laparoscopy and intraperitoneal chemotherapy (MMC 20 mg on day 1; CDDP 20 mg on days 1-5) before surgical resection or systemic chemotherapy (docetaxel 60 mg/m2 on day 1; CDDP 10 mg/m2 on days 1-5; 5-fluorouracil 350 mg/m2 on days 1-5), specimens of PLC were collected and were subjected to RT-PCR. All patients except for one who showed lower level of RT-PCR and finally revealed negative outcome, and all but one patient who showed an values level in the period of treatment died of recurrence, suggesting that evaluation of genetic changes using RT-PCR analysis can provide the practical information for detecting free cancer cells in the peritoneal cavity with high sensitivity and for selecting patients at high risk of peritoneal metastasis, leading to the prediction of chemotherapeutic efficacy for these patients.
Extensive intra-operative peritoneal lavage (EIPL) therapy, i.e. extensively repeated dilution and complete suction, serve as a very simple and non-aggressive prophylactic treatment for peritoneal metastasis of gastric cancer patients with peritoneal free cancer cells[72]. Yamamoto et al.[73] described that the peritoneal relapse rate of the patients with EIPL therapy was significantly lower than that of the patients without EIPL therapy. Although intra-peritoneal free cancer cells were detected immediately after curative surgery using RT-PCR analysis, no cancer cells were identified in the PLF after EIPL therapy[73]. A recent study using ultra-rapid quantitative RT-PCR has shown that the number of free cancer cells in PLF was serially diluted 3.8 × 105 ± 1.4 × 105 to 2.8 ± 1.5 cells/100 mL by 6 to 8 L of saline. Notably, CEA mRNA disappeared completely from the PLF after seven to nine washes. Intraperitoneal chemotherapy followed by EIPL using an ultra-rapid detection method may be acceptable for patients with free cancer cells in PLF after curative operation[74].
In a recent, the eligibility criteria for randomized controlled trials of neo-adjuvant chemotherapy or hyperthermic intraperitoneal chemotherapy in locally advanced gastric cancer included the presence of positive PLC at the staging laparoscopy[75]. Recently previous study presented 103 patients with gastric cancer who underwent staging laparoscopy and peritoneal metastasis was confirmed. Among them, 68 patients received the intravenous and intraperitoneal paclitaxel plus oral S-1 as induction chemotherapy. PLF of these patients was repetitively collected via intraperitoneal access ports. When a second laparoscopy showed negative PLC, gastrectomy was considered. Significant prolonged survival of patients with CmRI values that had once reduced to < 100 was identified by conversion gastrectomy. The OS of patients with a preoperative CmRI value < 100 was significantly improved compared with that of those with a preoperative CmRI value > 100 among patients who underwent conversion gastrectomy[37].
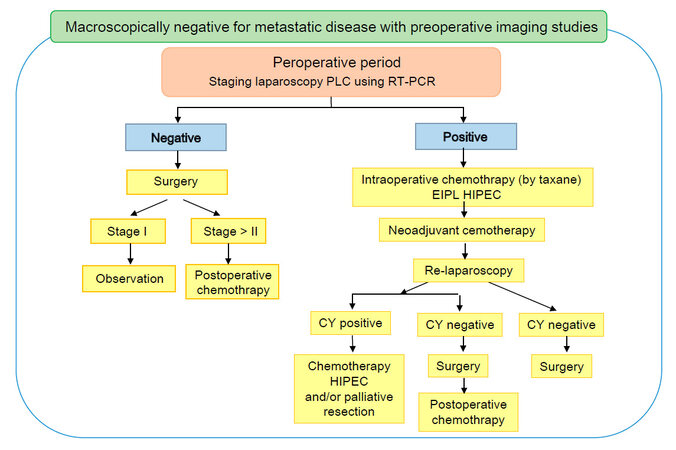
Based on these findings, we propose a treatment strategy for gastric cancer patients with positive PLC using RT-PCR in Figure 1.
Future perspectives of PLC by genetic techniques
Although numerous studies have presented that molecular analysis using RT-PCR may be useful for the detection of free cancer cells, there are still several obstacles for realizing the clinical application of genetically diagnosed PLC as a routine service. Namely, time-consuming, expensive, and relatively arduous techniques compared with conventional cytology are pointed out, and the sensitivity is broadly variable between laboratories; furthermore, procedures for quantitative assessment of free cancer cells are lacking. Recently, experimental studies proposing rapid, accurate, more standardized, and cost-effective detection methods have been reported. As described above, TRC can be a rapid and quantitative diagnostic technique to target CEA mRNA because it does not require cDNA synthesis and the reactions of amplification, and detection occur in a single tube, and take less than 1 h[36,76]. The reverse transcription-loop-mediated isothermal amplification (RT-LAMP) technique is a promising candidate to reduce the time requirement. In fact, there are several practical advantages to the RT-LAMP technique: it requires only simple reaction procedures, the compact incubator or turbidimeter equipment costs less than $5000, and needs less than 1 h to obtain the final results[10]. Among patients with negative cytology, those with a positive RT-LAMP reaction had a shorter survival than those with negative RT-LAMP reaction results. The RT-LAMP method may be an alternative method to determine the necessity or feasibility of surgery. Searching for a specific diagnostic marker for peritoneal metastasis by a rapid PCR method may help patients avoid redundant surgery and determine adequate preoperative chemotherapy.
As previously stated, numerous efforts have been made to gain the detection rate of intraperitoneal free cancer cells. The main purpose of these studies must principally be an enhancement of the sensitivity of PLC. To eliminate diagnostic errors and the misunderstanding of molecular diagnostic results for the sake of determining the best treatment plan, combined multiple markers would be practical for diagnosis of micrometastasis. Novel markers should also be sought. Multimarker PCR would be more clinically useful in getting expanded broad genetic profile in the near future, but this has yet to be investigated.
It is important to determine which genes should be analyzed for clinical decision making. Because personalized cancer genome analysis become more accepted and feasible, the genetic analysis of individual gastric tumors may provide insight into which tumor markers are the most sensitive for detection. Recently, The Cancer Genome Atlas Research Network (TCGA) advocated a novel classification system based on a genomic and molecular basis dividing gastric cancer into four major subtypes[77]. These sub-types include Epstein Barr Virus-infected tumors (EBV), microsatellite instability-associated tumors (MSI), genomically stable tumors (GS) and chromosomally unstable/chromosomal instability (CIN). EBV reveals mutations in PIK3CA and amplifications of JAK-2, PD-L1/2 as well as hypermethylation. MSI demonstrate multiple mutations including PIK3CA, ERBB3, HER2, EGFR in addition to MLH1 silencing. GS is related with CDH1 and RHOA mutations while CIN tumors harbor focal amplification of receptor tyrosine kinases in addition recurrent TP53 mutations. It is plausible that the relation of these genetic markers with peritoneal metastasis can be clarified on the basis of these molecular subtypes, which will lead to a future promising new candidate genetic markers in PLC for detecting intraperitoneal micrometastasis and a guide to new targeting agents. PLC should be considered as not just a survival predictor, but an important factor which can determine diagnosis and treatment of advanced gastric cancer after curative resection. Detection of molecular changes in PLF during chemotherapy, resulting in chemoresistance, could offer a promising way to shift the course of chemotherapy at the appropriate time as well as to find new therapeutic targets.
Conclusion
In conclusion, new genetic technologies are improving the detection of micrometastasis in the peritoneum, although conventional cytology is still the gold standard for PLC. The development of genetic PLC based on comprehensive genomic analysis could help us to identify patients who should be treated completely with multimodal therapy in addition to radical surgery, and will be very relevant to all sorts of clinical decision-making.
Declarations
AcknowledgmentsWe would like to thank our colleagues in our laboratory for helpful discussions. We apologize for not being able to cite all the relevant publications due to space limits.
Authors’ contributionsDesigned this review: Yashiro M, Matsuoka T
Wrote and edited the manuscript: Matsuoka T
Financial support and sponsorshipNone.
Conflicts of interestThere are no conflicts of interest.
Patient consentNot applicable.
Ethics approvalNot applicable.
Copyright© The Author(s) 2018.
REFERENCES
1. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends--an update. Cancer Epidemiol Biomarkers Prev 2016;25:16-27.
2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86.
3. Kagawa S, Shigeyasu K, Ishida M, Watanabe M, Tazawa H, Nagasaka T, Shirakawa Y, Fujiwara T. Molecular diagnosis and therapy for occult peritoneal metastasis in gastric cancer patients. World J Gastroenterol 2014;20:17796-803.
4. Bando E, Yonemura Y, Takeshita Y, Taniguchi K, Yasui T, Yoshimitsu Y, Fushida S, Fujimura T, Nishimura G, Miwa K. Intraoperative lavage for cytological examination in 1,297 patients with gastric carcinoma. Am J Surg 1999;178:256-62.
5. Higaki E, Yanagi S, Gotohda N, Kinoshita T, Kuwata T, Nagino M, Ochiai A, Fujii S. Intraoperative peritoneal lavage cytology offers prognostic significance for gastric cancer patients with curative resection. Cancer Sci 2017;108:978-86.
6. Kodera Y, Ito S, Mochizuki Y, Ohashi N, Tanaka C, Kobayashi D, Kojima H, Matsui T, Kondo K, Fujiwara M. Long-term follow up of patients who were positive for peritoneal lavage cytology: final report from the CCOG0301 study. Gastric Cancer 2012;15:335-7.
7. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1-19.
8. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
9. De Andrade JP, Mezhir JJ. The critical role of peritoneal cytology in the staging of gastric cancer: an evidence-based review. J Surg Oncol 2014;110:291-7.
10. Yoneda A, Taniguchi K, Torashima Y, Susumu S, Kanetaka K, Kuroki T, Eguchi S. The detection of gastric cancer cells in intraoperative peritoneal lavage using the reverse transcription--loop-mediated isothermal amplification method. J Surg Res 2014;187:e1-6.
11. Wong J, Kelly KJ, Mittra A, Gonen M, Allen P, Fong Y, Coit D. Rt-PCR increases detection of submicroscopic peritoneal metastases in gastric cancer and has prognostic significance. J Gastrointest Surg 2012;16:889-96; discussion 896.
12. Bentrem D, Wilton A, Mazumdar M, Brennan M, Coit D. The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann Surg Oncol 2005;12:347-53.
14. Burke EC, Karpeh MS, Conlon KC, Brennan MF. Laparoscopy in the management of gastric adenocarcinoma. Ann Surg 1997;225:262-7.
15. Wang JY, Lin SR, Lu CY, Chen CC, Wu DC, Chai CY, Chen FM, Hsieh JS, Huang TJ. Gastric cancer cell detection in peritoneal lavage: RT-PCR for carcinoembryonic antigen transcripts versus the combined cytology with peritoneal carcinoembryonic antigen levels. Cancer Lett 2005;223:129-35.
16. Kodera Y, Nakanishi H, Ito S, Mochizuki Y, Ohashi N, Yamamura Y, Fujiwara M, Koike M, Tatematsu M, Nakao A. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: analysis of real time reverse transcriptase-polymerase chain reaction after 5 years of followup. J Am Coll Surg 2006;202:231-6.
17. Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer 2014;17:26-33.
18. Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol 2014;40:584-91.
19. Asao T, Fukuda T, Yazawa S, Nagamachi Y. Carcinoembryonic antigen levels in peritoneal washings can predict peritoneal recurrence after curative resection of gastric cancer. Cancer 1991;68:44-7.
20. Hiroshige K, Iwamoto M, Kabashima N, Mutoh Y, Yuu K, Ohtani A. Prolonged use of intradialysis parenteral nutrition in elderly malnourished chronic haemodialysis patients. Nephrol Dial Transplant 1998;13:2081-7.
21. Liang HC, Dahan M, Karlin KD. Dioxygen-activating bio-inorganic model complexes. Curr Opin Chem Biol 1999;3:168-75.
22. Yamamoto M, Yoshinaga K, Matsuyama A, Tsutsui S, Ishida T. CEA/CA72-4 levels in peritoneal lavage fluid are predictive factors in patients with gastric carcinoma. J Cancer Res Clin Oncol 2014;140:607-12.
23. Riethdorf S, Wikman H, Pantel K. Review: biological relevance of disseminated tumor cells in cancer patients. Int J Cancer 2008;123:1991-2006.
24. Nakanishi H, Kodera Y, Torii A, Hirai T, Yamamura Y, Kato T, Kito T, Tatematsu M. Detection of carcinoembryonic antigen-expressing free tumor cells in peritoneal washes from patients with gastric carcinoma by polymerase chain reaction. Jpn J Cancer Res 1997;88:687-92.
25. Yonemura Y, Endou Y, Fujimura T, Fushida S, Bandou E, Kinoshita K, Sugiyama K, Sawa T, Kim BS, Sasaki T. Diagnostic value of preoperative RT-PCR-based screening method to detect carcinoembryonic antigen-expressing free cancer cells in the peritoneal cavity from patients with gastric cancer. ANZ J Surg 2001;71:521-8.
26. Fujii S, Kitayama J, Kaisaki S, Sasaki S, Seto Y, Tominaga O, Tsuno N, Umetani N, Yokota H, Kitamura K, Tsuruo T, Nagawa H. Carcinoembryonic antigen mRNA in abdominal cavity as a useful predictor of peritoneal recurrence of gastric cancer with serosal exposure. J Exp Clin Cancer Res 2002;21:547-53.
27. Tokuda K, Natsugoe S, Nakajo A, Miyazono F, Ishigami S, Hokita S, Takao S, Eizuru Y, Aikou T. Clinical significance of CEA-mRNA expression in peritoneal lavage fluid from patients with gastric cancer. Int J Mol Med 2003;11:79-84.
28. Ueno H, Yoshida K, Hirai T, Kono F, Kambe M, Toge T. Quantitative detection of carcinoembryonic antigen messenger RNA in the peritoneal cavity of gastric cancer patients by real-time quantitative reverse transcription polymerase chain reaction. Anticancer Res 2003;23:1701-8.
29. Oyama K, Terashima M, Takagane A, Maesawa C. Prognostic significance of peritoneal minimal residual disease in gastric cancer detected by reverse transcription-polymerase chain reaction. Br J Surg 2004;91:435-43.
30. Ito S, Nakanishi H, Kodera Y, Mochizuki Y, Tatematsu M, Yamamura Y. Prospective validation of quantitative CEA mRNA detection in peritoneal washes in gastric carcinoma patients. Br J Cancer 2005;93:986-92.
31. Fujiwara Y, Doki Y, Taniguchi H, Sohma I, Takiguchi S, Miyata H, Yamasaki M, Monden M. Genetic detection of free cancer cells in the peritoneal cavity of the patient with gastric cancer: present status and future perspectives. Gastric Cancer 2007;10:197-204.
32. Nakanishi H, Kodera Y, Yamamura Y, Ito S, Kato T, Ezaki T, Tatematsu M. Rapid quantitative detection of carcinoembryonic antigen-expressing free tumor cells in the peritoneal cavity of gastric-cancer patients with real-time RT-PCR on the lightcycler. Int J Cancer 2000;89:411-7.
33. Kodera Y, Isobe K, Yamauchi M, Satta T, Hasegawa T, Oikawa S, Kondoh K, Akiyama S, Itoh K, Nakashima I. Expression of carcinoembryonic antigen (CEA) and nonspecific crossreacting antigen (NCA) in gastrointestinal cancer; the correlation with degree of differentiation. Br J Cancer 1993;68:130-6.
34. Kodera Y, Nakanishi H, Ito S, Yamamura Y, Kanemitsu Y, Shimizu Y, Hirai T, Yasui K, Kato T, Tatematsu M. Quantitative detection of disseminated cancer cells in the greater omentum of gastric carcinoma patients with real-time RT-PCR: a comparison with peritoneal lavage cytology. Gastric Cancer 2002;5:69-76.
35. Ohashi N, Nakanishi H, Kodera Y, Ito S, Mochizuki Y, Koike M, Fujiwara M, Yamamura Y, Tatematsu M, Nakao A, Kato T. Intraoperative quantitative detection of CEA mRNA in the peritoneal lavage of gastric cancer patients with transcription reverse-transcription concerted (TRC) method. A comparative study with real-time quantitative RT-PCR. Anticancer Res 2007;27:2769-77.
36. Fujiwara Y, Okada K, Hanada H, Tamura S, Kimura Y, Fujita J, Imamura H, Kishi K, Yano M, Miki H, Okada K, Takayama O, Aoki T, Mori M, Doki Y. The clinical importance of a transcription reverse-transcription concerted (TRC) diagnosis using peritoneal lavage fluids in gastric cancer with clinical serosal invasion: a prospective, multicenter study. Surgery 2014;155:417-23.
37. Yamaguchi H, Satoh Y, Ishigami H, Kurihara M, Yatomi Y, Kitayama J. Peritoneal lavage CEA mRNA levels predict conversion gastrectomy outcomes after induction chemotherapy with intraperitoneal paclitaxel in gastric cancer patients with peritoneal metastasis. Ann Surg Oncol 2017;24:3345-52.
38. Kodera Y, Nakanishi H, Ito S, Yamamura Y, Fujiwara M, Koike M, Hibi K, Ito K, Tatematsu M, Nakao A. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: detection of cytokeratin 20 mRNA in peritoneal washes, in addition to detection of carcinoembryonic antigen. Gastric Cancer 2005;8:142-8.
39. Sugita Y, Fujiwara Y, Taniguchi H, Mori T, Ishii T, Niwa H, Okada Y, Takiguchi S, Yasuda T, Yano M, Monden M. Quantitative molecular diagnosis of peritoneal lavage fluid for prediction of peritoneal recurrence in gastric cancer. Int J Oncol 2003;23:1419-23.
40. Katsuragi K, Yashiro M, Sawada T, Osaka H, Ohira M, Hirakawa K. Prognostic impact of PCR-based identification of isolated tumour cells in the peritoneal lavage fluid of gastric cancer patients who underwent a curative R0 resection. Br J Cancer 2007;97:550-6.
41. Takata A, Kurokawa Y, Fujiwara Y, Nakamura Y, Takahashi T, Yamasaki M, Miyata H, Nakajima K, Takiguchi S, Mori M, Doki Y. Prognostic value of CEA and CK20 mRNA in the peritoneal lavage fluid of patients undergoing curative surgery for gastric cancer. World J Surg 2014;38:1107-11.
42. Yang B, O'Herrin SM, Wu J, Reagan-Shaw S, Ma Y, Bhat KM, Gravekamp C, Setaluri V, Peters N, Hoffmann FM, Peng H, Ivanov AV, Simpson AJ, Longley BJ. MAGE-A, mMage-b, and MAGE-C proteins form complexes with KAP1 and suppress p53-dependent apoptosis in MAGE-positive cell lines. Cancer Res 2007;67:9954-62.
43. Jeon CH, Kim IH, Chae HD. Prognostic value of genetic detection using CEA and MAGE in peritoneal washes with gastric carcinoma after curative resection: result of a 3-year follow-up. Medicine (Baltimore) 2014;93:e83.
44. Li J, Yang Y, Fujie T, Tanaka F, Mimori K, Haraguchi M, Ueo H, Mori M, Akiyoshi T. Expression of the MAGE gene family in human gastric carcinoma. Anticancer Res 1997;17:3559-63.
45. Hiraki M, Kitajima Y, Koga Y, Tanaka T, Nakamura J, Hashiguchi K, Noshiro H, Miyazaki K. Aberrant gene methylation is a biomarker for the detection of cancer cells in peritoneal wash samples from advanced gastric cancer patients. Ann Surg Oncol 2011;18:3013-9.
46. Di Leva G, Croce CM. The role of microRNAs in the tumorigenesis of ovarian cancer. Front Oncol 2014;3:153.
47. Tokuhisa M, Ichikawa Y, Kosaka N, Ochiya T, Yashiro M, Hirakawa K, Kosaka T, Makino H, Akiyama H, Kunisaki C, Endo I. Exosomal miRNAs from peritoneum lavage fluid as potential prognostic biomarkers of peritoneal metastasis in gastric cancer. PLoS One 2015;10:e0130472.
48. Radke S, Pirkmaier A, Germain D. Differential expression of the F-box proteins Skp2 and Skp2B in breast cancer. Oncogene 2005;24:3448-58.
49. Miwa T, Kanda M, Tanaka H, Tanaka C, Kobayashi D, Umeda S, Iwata N, Hayashi M, Yamada S, Fujii T, Fujiwara M, Kodera Y. FBXO50 enhances the malignant behavior of gastric cancer cells. Ann Surg Oncol 2017;24:3771-9.
50. Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med (Maywood) 2006;231:20-7.
51. Li Z, Zhang D, Zhang H, Miao Z, Tang Y, Sun G, Dai D. Prediction of peritoneal recurrence by the mRNA level of CEA and MMP-7 in peritoneal lavage of gastric cancer patients. Tumour Biol 2014;35:3463-70.
52. Mirigian LS, Makareeva E, Koistinen H, Itkonen O, Sorsa T, Stenman UH, Salo T, Leikin S. Collagen degradation by tumor-associated trypsins. Arch Biochem Biophys 2013;535:111-4.
53. Fujimura T, Ohta T, Kitagawa H, Fushida S, Nishimura GI, Yonemura Y, Elnemr A, Miwa K, Nakanuma Y. Trypsinogen expression and early detection for peritoneal dissemination in gastric cancer. J Surg Oncol 1998;69:71-5.
54. Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma L, Xue X, Wei G, Liu X, Fang G. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun 2008;374:533-7.
55. Iida T, Iwahashi M, Katsuda M, Ishida K, Nakamori M, Nakamura M, Naka T, Ojima T, Ueda K, Hayata K, Yasuoka H, Yamaue H. Prognostic significance of IL-17 mRNA expression in peritoneal lavage in gastric cancer patients who underwent curative resection. Oncol Rep 2014;31:605-12.
56. Bernardes de Jesus B, Blasco MA. Telomerase at the intersection of cancer and aging. Trends Genet 2013;29:513-20.
57. Da MX, Wu XT, Guo TK, Zhao ZG, Luo T, Qian K, Zhang MM, Wang J. Clinical significance of telomerase activity in peritoneal lavage fluid from patients with gastric cancer and its relationship with cellular proliferation. World J Gastroenterol 2007;13:3122-7.
58. Sakakura C, Hagiwara A, Nakanishi M, Shimomura K, Takagi T, Yasuoka R, Fujita Y, Abe T, Ichikawa Y, Takahashi S, Ishikawa T, Nishizuka I, Morita T, Shimada H, Okazaki Y, Hayashizaki Y, Yamagishi H. Differential gene expression profiles of gastric cancer cells established from primary tumour and malignant ascites. Br J Cancer 2002;87:1153-61.
59. Gilbert J, Haber M, Bordow SB, Marshall GM, Norris MD. Use of tumor-specific gene expression for the differential diagnosis of neuroblastoma from other pediatric small round-cell malignancies. Am J Pathol 1999;155:17-21.
60. Sakakura C, Takemura M, Hagiwara A, Shimomura K, Miyagawa K, Nakashima S, Yoshikawa T, Takagi T, Kin S, Nakase Y, Fujiyama J, Hayasizaki Y, Okazaki Y, Yamagishi H. Overexpression of dopa decarboxylase in peritoneal dissemination of gastric cancer and its potential as a novel marker for the detection of peritoneal micrometastases with real-time RT-PCR. Br J Cancer 2004;90:665-71.
61. Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 2009;27:1006-20.
62. Horikawa M, Iinuma H, Inoue T, Ogawa E, Fukushima R. Clinical significance of intraperitoneal CD44 mRNA levels of magnetically separated CD45-negative EpCAM-positive cells for peritoneal recurrence and prognosis in stage II and III gastric cancer patients. Oncol Rep 2011;25:1413-20.
63. Bachelor MA, Lu Y, Owens DM. L-3-Phosphoserine phosphatase (PSPH) regulates cutaneous squamous cell carcinoma proliferation independent of L-serine biosynthesis. J Dermatol Sci 2011;63:164-72.
64. Shimomura K, Sakakura C, Takemura M, Takagi T, Fukuda K, Kin S, Nakase Y, Miyagawa K, Ohgaki M, Fujiyama J, Fujita Y, Nakanishi M, Hagiwara A, Shirane M, Okazaki Y, Hayashizaki Y, Yamagishi H. Combination of L-3-phosphoserine phosphatase and CEA using real-time RT-PCR improves accuracy in detection of peritoneal micrometastasis of gastric cancer. Anticancer Res 2004;24:1113-20.
65. Hippo Y, Yashiro M, Ishii M, Taniguchi H, Tsutsumi S, Hirakawa K, Kodama T, Aburatani H. Differential gene expression profiles of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Res 2001;61:889-95.
66. Mori T, Fujiwara Y, Sugita Y, Azama T, Ishii T, Taniguchi K, Yamazaki K, Takiguchi S, Yasuda T, Yano M, Monden M. Application of molecular diagnosis for detection of peritoneal micrometastasis and evaluation of preoperative chemotherapy in advanced gastric carcinoma. Ann Surg Oncol 2004;11:14-20.
67. Kodera Y, Ito S, Mochizuki Y, Kondo K, Koshikawa K, Suzuki N, Kojima H, Kojima T, Matsui T, Takase T, Tsuboi K, Fujiwara M, Nakao A. A phase II study of radical surgery followed by postoperative chemotherapy with S-1 for gastric carcinoma with free cancer cells in the peritoneal cavity (CCOG0301 study). Eur J Surg Oncol 2009;35:1158-63.
68. Kurebayashi J, Nukatsuka M, Fujioka A, Saito H, Takeda S, Unemi N, Fukumori H, Kurosumi M, Sonoo H, Dickson RB. Postsurgical oral administration of uracil and tegafur inhibits progression of micrometastasis of human breast cancer cells in nude mice. Clin Cancer Res 1997;3:653-9.
69. Yokoyama H, Nakanishi H, Kodera Y, Ikehara Y, Ohashi N, Ito Y, Koike M, Fujiwara M, Tatematsu M, Nakao A. Biological significance of isolated tumor cells and micrometastasis in lymph nodes evaluated using a green fluorescent protein-tagged human gastric cancer cell line. Clin Cancer Res 2006;12:361-8.
70. Ito S, Kodera Y, Mochizuki Y, Kojima T, Nakanishi H, Yamamura Y. Phase II clinical trial of postoperative S-1 monotherapy for gastric cancer patients with free intraperitoneal cancer cells detected by real-time RT-PCR. World J Surg 2010;34:2083-9.
71. Kodera Y. Gastric cancer with minimal peritoneal metastasis: is this a sign to give up or to treat more aggressively? Nagoya J Med Sci 2013;75:3-10.
72. Shimada S, Tanaka E, Marutsuka T, Honmyo U, Tokunaga H, Yagi Y, Aoki N, Ogawa M. Extensive intraoperative peritoneal lavage and chemotherapy for gastric cancer patients with peritoneal free cancer cells. Gastric Cancer 2002;5:168-72.
73. Yamamoto K, Shimada S, Hirota M, Yagi Y, Matsuda M, Baba H. EIPL (extensive intraoperative peritoneal lavage) therapy significantly reduces peritoneal recurrence after pancreatectomy in patients with pancreatic cancer. Int J Oncol 2005;27:1321-8.
74. Marutsuka T, Shimada S, Shiomori K, Hayashi N, Yagi Y, Yamane T, Ogawa M. Mechanisms of peritoneal metastasis after operation for non-serosa-invasive gastric carcinoma: an ultrarapid detection system for intraperitoneal free cancer cells and a prophylactic strategy for peritoneal metastasis. Clin Cancer Res 2003;9:678-85.
75. Ji ZH, Peng KW, Li Y. Intraperitoneal free cancer cells in gastric cancer: pathology of peritoneal carcinomatosis and rationale for intraperitoneal chemotherapy/hyperthermic intraperitoneal chemotherapy in gastric cancer. Transl Gastroenterol Hepatol 2016;1:69.
76. Ishii T, Fujiwara Y, Ohnaka S, Hayashi T, Taniguchi H, Takiguchi S, Yasuda T, Yano M, Monden M. Rapid genetic diagnosis with the transcription-reverse transcription concerted reaction system for cancer micrometastasis. Ann Surg Oncol 2004;11:778-85.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Matsuoka T, Yashiro M. Significance of peritoneal lavage cytology based on genetic signatures in gastric cancer. J Cancer Metastasis Treat 2018;4:6. http://dx.doi.org/10.20517/2394-4722.2017.85
AMA Style
Matsuoka T, Yashiro M. Significance of peritoneal lavage cytology based on genetic signatures in gastric cancer. Journal of Cancer Metastasis and Treatment. 2018; 4: 6. http://dx.doi.org/10.20517/2394-4722.2017.85
Chicago/Turabian Style
Matsuoka, Tasuku, Masakazu Yashiro. 2018. "Significance of peritoneal lavage cytology based on genetic signatures in gastric cancer" Journal of Cancer Metastasis and Treatment. 4: 6. http://dx.doi.org/10.20517/2394-4722.2017.85
ACS Style
Matsuoka, T.; Yashiro M. Significance of peritoneal lavage cytology based on genetic signatures in gastric cancer. J. Cancer. Metastasis. Treat. 2018, 4, 6. http://dx.doi.org/10.20517/2394-4722.2017.85
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 1 clicks
Cite This Article 1 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.