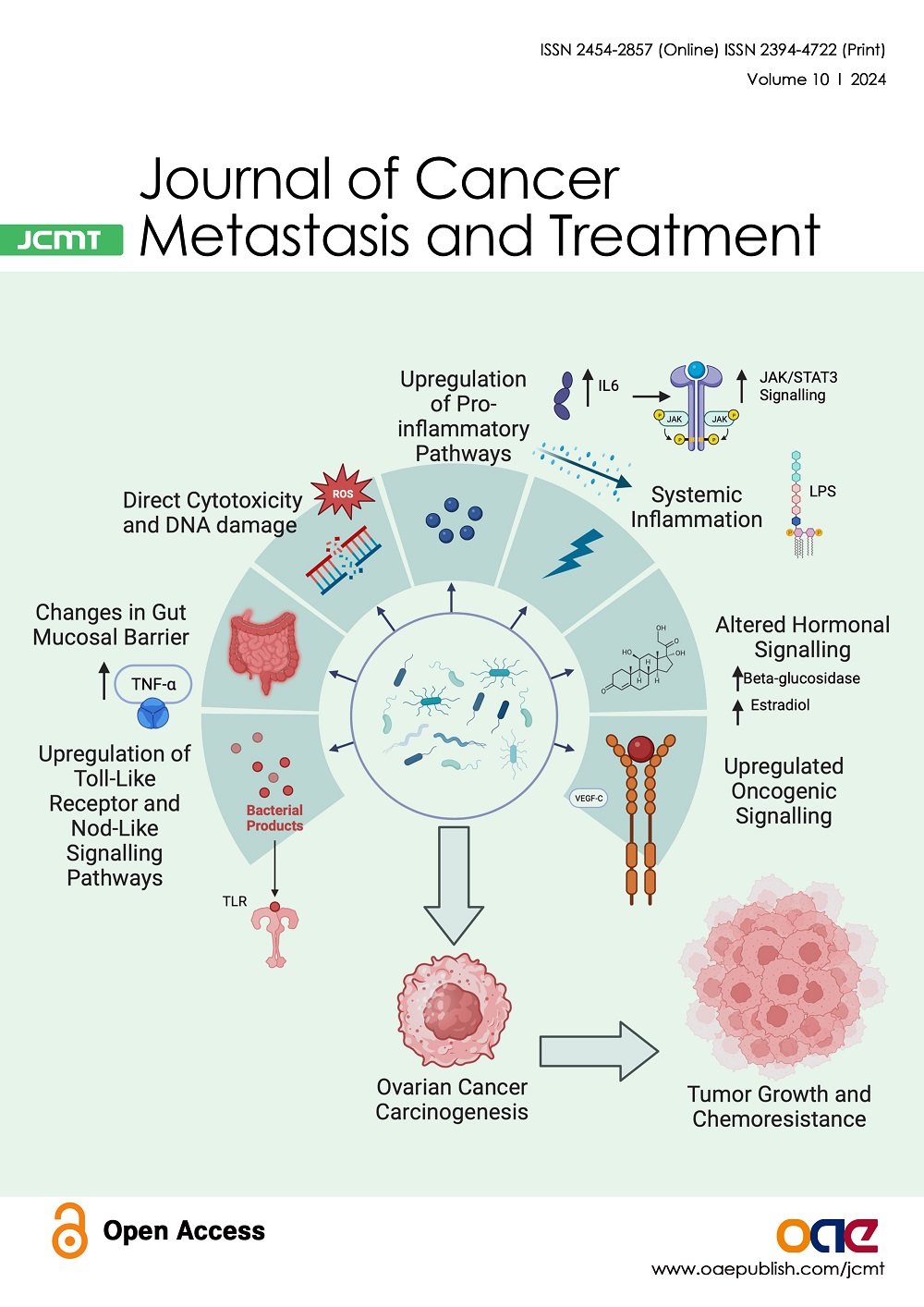
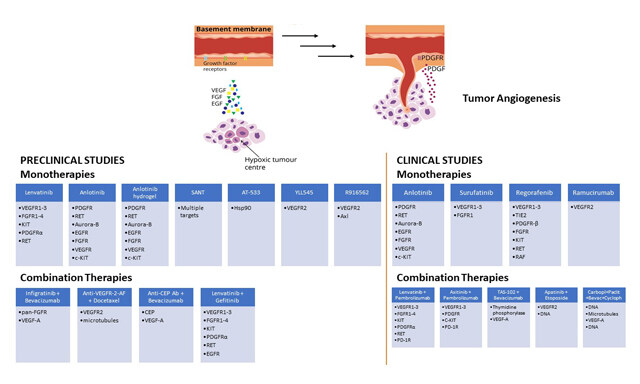
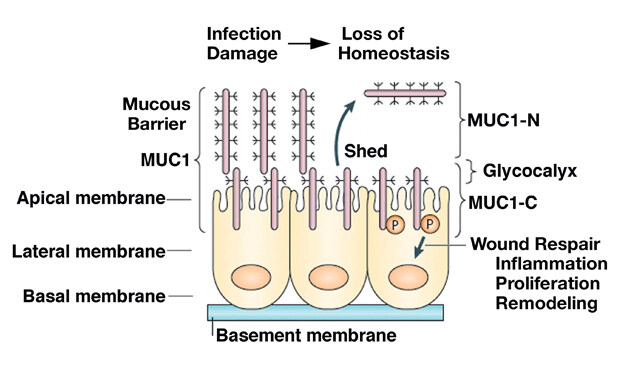
Journal of Cancer Metastasis and Treatment
Views: Downloads:
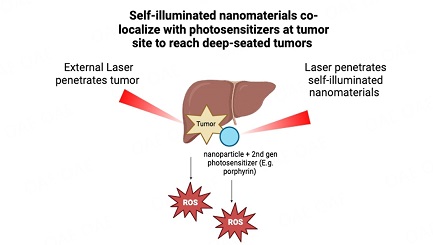
Views: Downloads:
Views: Downloads:
Views: Downloads:
Views: Downloads:
Views: Downloads:
Views: Downloads:
Views: Downloads:
Data
2252
Authors
1359
Reviewers
2015
Published Since
3,313,806
Article Views
561,203
Article Downloads
For Reviewers
For Readers
Add your e-mail address to receive forthcoming Issues of this journal:
Themed Collections
Cancer Treatment
Metastasis
Microenvironment
Immunotherapy
Breast Cancer Metastasis
Bone Metastasis
Metastatic Renal Cell Carcinoma
Lymph node metastasis
Mesothelioma
Hematological Malignancies
Thyroid Cancer
Liquid Biopsies
Early Diagnosis
Lung Cancer
Brain Tumors
Lymphoma
Oncolytic Virus
Cervical Cancer
Cancer Stem Cells
Related Journals
Related Journals
Data
2252
Authors
1359
Reviewers
2015
Published Since
3,313,806
Article Views
561,203
Article Downloads