Leptomeningeal metastases from non-small cell lung cancer: state of the art and recent advances
Abstract
Patients with leptomeningeal metastases (LM) from non-small cell lung cancer (NSCLC) have a poor outcome with survival of less than 1 year regardless of advancements in treatment strategy. In the past, some randomized clinical trials have been conducted with heterogeneous inclusion criteria, diagnostic parameters, response evaluation and primary endpoints. Efforts to develop a standardized magnetic resonance imaging (MRI) assessment and liquid biopsy techniques to monitor disease evolution in plasma or cerebrospinal fluid (CSF) are underway. This review aims to cover the main clinical and diagnostic challenges of LM from NSCLC, in particular the role of MRI, CSF cytology and liquid biopsy for the diagnosis and monitoring of the disease, as well as the most recent clinical trials on targeted therapies. Targeted therapy, such as epidermal growth factor receptor tyrosine kinase inhibitors and anaplastic lymphoma kinase rearranged inhibitors, represent a feasible treatment with encouraging results in terms of disease control and survival. For ineligible patients, immune checkpoint inhibitors could represent a therapeutic option with acceptable tolerance, although clinical trials focused on LM from NSCLC are lacking and represent a research focus for the future.
Keywords
Introduction
Leptomeningeal metastases (LM) represent an end-stage complication of advanced systemic cancer in approximately 5% of patients. Autopsy series have revealed a high prevalence of undiagnosed or asymptomatic LM in 19% of patients with solid tumors[1], representing the third most common metastatic complication of the nervous system after brain metastases (BM) and epidural metastases. The incidence of LM in patients with cancer is increasing due to better tools for diagnosis and monitoring and more effective targeted therapies that lead to prolonged survival[2]. However, overall survival remains in the order of weeks to months regardless of treatment type.
A recent cohort study of 163 patients with LM has shown a median age of 57 years, and LM was the initial presentation of cancer in 19 patients (11.7%), while in 28 patients (19%) LM was diagnosed during the initial treatment course of primary tumor, and in the remaining 116 patients (81%) LM was diagnosed at recurrence. The most common primary solid tumors in this cohort are non-small cell lung adenocarcinoma (NSCLC - 52%), followed by small cell lung carcinoma (SCLC - 18%), and breast cancer (16%)[3].
NSCLC has a significant risk LM (20% of patients)[4] with a median development time of 12 months (range 2-18 months) from diagnosis of the primary tumor[3]. Epidermal growth factor receptor (EGFR) mutated and anaplastic lymphoma kinase (ALK) gene rearranged NSCLC are more prone to recur with LM[5,6]. Thus, there is need to improve diagnostic tools, validate biomarkers to monitor disease progression, and search for new treatment regimens for LM patients.
Here, we review the clinical and diagnostic challenges of LM from NSCLC, the role of magnetic resonance imaging (MRI), cerebrospinal fluid (CSF) cytology and liquid biopsy, as well as the most recent clinical trials on targeted therapies.
The role of CNS barriers in drug delivery
The CNS is considered as a sanctuary site which is protected by different barriers from neurotoxic agents. The blood-brain barrier (BBB) consists of tight junctions (TJs) linked to the endothelial cells of the brain, creating a physical barrier that limits the passage of molecules[7]. The specialized endothelial cells maintain a continuous, non-fenestrated basal lamina and interact with other perivascular cells, such as astrocytes, pericytes, and perivascular macrophages, which contribute to the integrity of the BBB[8]. Molecules may cross the BBB by two mechanisms. The paracellular transport consists of a diffuse and passive flow between the endothelial cells, and is regulated by physicochemical properties, including molecular weight, electrical charge, and lipophilicity. In general, the TJs reduce the paracellular transport of molecules when the BBB is intact; thus, the paracellular transport is limited to small, lipophilic molecules that are less than 500 Daltons[9]. The transcellular transport consists of a flow of molecules across the luminal side of the endothelial cell, through the cytoplasm, and then to the abluminal side into the brain interstitium. Some active transport mechanisms are typical of transcellular transport for larger and less lipophilic molecules, such as glucose, insulin, albumin, blood cells, infectious agents, and potential neurotoxins[10].
Similarly, the BBB impacts the ability of therapeutic agents to penetrate the CNS. In fact, more than 90% of all small-molecules, and nearly 100% of large compounds, have poor penetration through the BBB[11], leading to a decreased efficacy on CNS disease control from chemotherapy and targeted agents. Notably, the BBB is normal in pre-metastatic niche and micrometastases (< 1 mm), and protects them from most of anticancer agents that are employed in the adjuvant treatment of NSCLC[12,13]. The most recent generations of anaplastic lymphoma kinase (ALK) inhibitors and EGFR tyrosine kinase inhibitors (TKIs) display an increased ability to cross the BBB, reaching significant CSF concentrations [Table 1], but these drugs may be actively transported back into the cerebral blood flow by efflux pumps. The most important is the P-glycoprotein 1 (P-gp1), a member of the ATP binding cassette family, which recognizes a wide range of compounds employed in the adjuvant setting of NSCLC (with the exception of AZD3759 and alectinib), and contribute to drug resistance[24].
CSF concentration and penetration of targeted agents in LM from NSCLC
| Drug | Target | Dosage/day | CSF level (nmol/L) | Penetration (CSF/plasma) % |
|---|---|---|---|---|
| First generation TKIs | ||||
| Erlotinib[6,14] | EGFR | 150 mg | 66.9 | 2.8-3.3 |
| Gefitinib[6] | EGFR | 250 mg | 8.2 | 1.13 |
| Second-generation TKIs | ||||
| Afatinib[15] | EGFR | 40 mg/m2 | 1.0 | 1.65 |
| Third-generation TKIs | ||||
| Osimertinib[16,17] | EGFR mutated Thr790Met | 160 mg | 7.51 | 2.5-16.0 |
| AZD3759[18] | EGFR | 300 mg bid | 25.2 | 100.0 |
| First-generation inhibitors | ||||
| Crizotinib[19] | ALK, MET, ROS1 | 250 mg | 0.14 | 0.26 |
| Second-generation inhibitors | ||||
| Ceritinib[20] | ALK, ROS1 | 450-600 mg | NA | 15.0 |
| Alectinib[21,22] | ALK, RET | 60 mg/kg | 2.69 | 63.0-94.0 |
| Brigatinib[20] | ALK, ROS1, EGFR | 90-180 mg | NA | NA |
| Third-generation inhibitors | ||||
| Lorlatinib[23] | ALK, ROS1 | 100 mg | 6.5-308 | 31.0-96.0 |
The blood-tumor barrier (BTB) lacks TJs and astrocyte-endothelial contacts, but is enriched with P-gp1 along the luminal and plasma membranes of tumor cells compared with BBB, and contributes to limiting the drug penetration on BM and LM[13]. More importantly, the permeability of BTB and BBB in BM and LM varies widely between lesions and regions of concern, resulting in non-homogeneous drug distribution[13,24,25].
The space between the CSF and the CSF-producing choroid plexus is known as the blood-CSF barrier, which determines the adequate concentration of molecules by primarily active transports[26]. Since the BBB and blood-CSF barrier use non comparable active transport mechanisms, and CSF drug concentrations strictly depend on blood-CSF barrier, the distribution of drug into CSF cannot be considered as a reliable measure of BBB permeability or surrogate of drug concentration in BM or LM[27]. With this regard, future phase 0 trials are strongly encouraged to analyze drug-target effects and pharmacokinetic-pharmacodynamic relationships in the early clinical development of new drugs[28]. Lastly, drug concentrations in the CSF are correlated with the free drug concentration in plasma: clinicians may modulate the concentration of free drug in plasma by changing the dose and schedules of treatment, including the use of high-dose or pulse administration[29-32].
Pathogenesis of leptomeningeal metastases from NSCLC
Tumor cells may reach the leptomeninges in different ways, such as hematogenous spread through the vessels of the arachnoid and choroid plexi, along peripheral nervous system by nerve and vascular sheaths, through lymphatic dissemination or invasion by contiguity[33]. Furthermore, access of the ventricular system or using a piecemeal compared with en-block tumor resection, have been suggested as risk factors for leptomeningeal dissemination[34,35]. Post-operative SRS is an effective adjunct to reduce the risk of local recurrence[36]. However, some studies have suggested that SRS may also be associated with increased rates of LM recurrence, with reported incidence of up to 31% in 1 year[37,38].
Complement component 3 (C3), which is produced by cancer cells in the CSF, is upregulated in LM models from lung and breast cancer. In particular, C3 promotes the disruption of blood-CSF barrier, leading to the passage of some mitogens, such as amphiregulin, that promotes tumor cell growth within leptomeninges[39]. Similarly, Matrix Metalloproteases (MMPs) type 9, A Disintegrin and Metalloproteases (ADAMs) type 8 and 17 interfere with the integrity of the blood-CSF barrier, facilitating the passage of tumor cells into the subarachnoid space[40]. Some driver mutations select clonal tumor cells making them more prone to metastasize to the CNS. Brastianos et al.[41] showed that distinct genetic alterations were not detected in the matched primary-tumor sample in 53% of 86 patients with BM, while spatially and temporally separated BM were genetically homogenous and shared similar druggable pathways, including PI3K/AKT/mTOR, CDK, and HER2/EGFR. Further investigations have revealed three regions with significantly higher amplification in BM from NSCLC, including MYC, YAP1, and MMP13, and deletions in CDKN2A/B[42]. Thus, the response to targeted therapies in BM or LM not necessarily recapitulate the response of the primary tumor: profiling the BM and LM might be advantageous in planning therapeutic interventions, predicting response, and discovering new targets that could be absent in the primary disease.
The acquired resistance to first-generation targeted therapy in NSCLC has been suggested to promote LM. Nanjo et al.[43] described that acquired resistance to gefitinib is characterized by an upregulation of MET and absence of T790M mutation. Moreover, the T790M mutation has not been identified neither in BM or LM[44,45], nor in CSF of patients who have developed LM following EGFR TKIs[46]. Jiang et al.[47] reported a lower frequency of T790M mutation (21%) and a higher frequency of MET amplification (39%) in the CSF, suggesting that MET amplification could confer a major risk of leptomeningeal invasion[48].
Clinical and radiological diagnosis of leptomeningeal metastases
Symptoms from LM are typically multifocal due to the involvement of different segments of the neuroaxis. Spinal cord and nerve roots are the most frequent sites of LM (60%), followed by cranial nerves (35%) and cerebrum (15%)[49]. Table 2 summarizes the most common symptoms from LM. As any site in the CNS may be involved, the evaluation of a patient with suspected LM is difficult, and signs and symptoms may be shared by BM, or mimic treatment-related toxicities or neurological paraneoplastic syndromes. Therefore, the neurological examination is crucial and should be performed by an expert neurologist. However, LM is a complication of solid tumors that is primarily being treated by medical oncologists, thus a standardized clinical evaluation is needed especially during follow up. The Neurologic Assessment in Neuro-Oncology (NANO) scale was drafted by a group of physicians, including neurologists, medical oncologists, radiation oncologists, and neurosurgeons, with expertise in neuro-oncology, and was tested during routine examination, reporting an objective clinician-reported outcome of neurologic function with high inter-observer agreement[50,51]. Unfortunately, the NANO scale is not useful enough, due to the low sensitivity to detect the multilevel involvement of the CNS typically seen in LM. With this regard, the Leptomeningeal Assessment for Neuro-Oncology (LANO) group has developed a standardized assessment with several domains, such as gait, strength, sensation, vision, eye movement, facial strength, hearing, swallowing, level of consciousness and behavior, which may be graded as 0 (normal), 1 (slight abnormal), 2 (moderate abnormal) and 3 (severe abnormal)[52]. However, the LANO scale needs to be validated, and other tools are being used to improve the diagnosis of LM.
Most common clinical manifestations of LM
| Headache | Related to an increased intracranial pressure, blockage of the CSF flow and obstructive hydrocephalus |
| Spinal symptoms | Lower motor neuron weakness, sensory loss, radicular and back/neck pain, bladder, sexual and bowel dysfunctions |
| Cranial nerve palsies | Diplopia and visual impairment (II), ophthalmoparesis (III-IV-VI), hearing loss (VIII), facial weakness (VII), trigeminal sensory impairment (V), dysphagia (XI-X) |
| Impaired consciousness | Mood and mental changes (especially in case of encephalopathy), seizures |
| Gait disturbances | Due to cerebellar (coupled with nystagmus, dysmetria, dysarthria) os sensitive ataxia |
| Nausea/vomiting | involvement of the vestibular nerve and floor of the fourth ventricle floor |
Brain and spinal cord MRI with contrast enhancement is mandatory for the assessment of suspected LM[53]. Contrast-enhanced T1-weighted and fluid-attenuated inversion recovery (FLAIR) sequences are the most sensitive to show LM[54]. Linear or nodular enhancing lesions of the cranial nerves and spinal nerve roots (e.g., cauda equina), brain sulci and cerebellar folia are the most common findings[55] [Figures 1 and 2]. Nodular lesions typically are small (< 5 mm) with a complex geometry, and to measure the tumor burden is difficult. As a result, the RANO Leptomeningeal Metastasis Group proposed a LANO scorecard for diagnosis of MRI in LM patients, but most of the raters experienced problems with the instructions on the scorecard, and discordance for the rating of single items at baseline and follow-up was observed. A new simplified RANO-LM score is now under development[56]. Similarly, the European Association for Neuro-Oncology and the European Society of Medical Oncology (EANO-ESMO) have proposed a classification of the radiological findings in LM: linear lesions (type A), nodular lesions (type B), both linear and nodular lesions (type C), absence of enhancing lesions in presence of hydrocephalus (type D)[57]. Overall, both LANO and EANO/ESMO groups have proposed a tentative diagnostic workup that include clinical symptoms, imaging, and CSF cytology for diagnosis and assessment of treatment response for LM; however, a major issue is to define measurable versus non-measurable lesions, and changes in the measurement that qualify for response. Due to these caveats, their application in daily clinical practice remains limited.
Figure 1. Linear enhancement of left temporal sulci from epidermal growth factor receptor mutated non-small-cell lung cancer
Figure 2. Diffuse linear spinal leptomeningeal enhancement from epidermal growth factor receptor and anaplastic lymphoma kinase wild-type non-small-cell lung cancer
In general, the sensitivity of contrast-enhanced MRI in detecting LM is about 70%-85% with a specificity of approximately 75%-90%[58]. Freilich et al.[59] have shown that contrast-enhanced MRI is altered in approximately 90% of patients with LM from solid tumors and positive CSF, while about 20%-30% of patients with LM may present a false-negative MRI[4]. Therefore, a negative MRI does not exclude a diagnosis of LM in a patient with typical neurological symptoms. No other alternative imaging techniques have been used to validate negative MRI results in cases of high index of suspicion of LM. For instance, there are no studies on the sensitivity and specificity of 18F-fluorodeoxyglucose positron emission tomography–computed tomography (FDG-PET-CT) for LM diagnosis, due to resolution issues. In one patient only a LM from NSCLC was detected using FDG-PET-CT[60].
CSF analysis
Some biochemical alterations may be found in the CSF of LM patients, such as an increased pressure (> 200 mm H2O) in 21%-42% of patients, high level of proteins (> 50 mg/dL) in 56%-91%, decreased level of glucose (<60 mg/dL), and elevated leucocyte count (> 4/mm3) in 48%-77.5%[61]. All these findings are not pathognomonic of LM, and a CSF cytology positive for neoplastic cells remains the gold standard for diagnosis. CSF cytology does not allow a quantitative analysis and has a low sensitivity with 30%-50% of LM patients with negative CSF[57]. In case of negative results after the first CSF tap, the EANO/ESMO Guidelines recommend performing a second lumbar puncture to improve the sensitivity up to 80%. Moreover, there is no evidence that a conversion to a negative CSF is correlated with disease control in leptomeninges and with a prolonged progression-free survival (PFS); thus, the cytological clearance of CSF is not a reliable method to monitor leptomeningeal response.
Liquid biopsy in leptomeningeal metastases from NSCLC
Liquid biopsy consists of detecting tumor biomarkers in body fluids, such as blood, plasma, CSF, urine, saliva, ascites, with the aim to diagnose and monitor disease. Different biomarkers may be detected, including circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), or exosomes. CSF liquid biopsy has been suggested as a more sensitive tool to achieve a diagnosis of LM than conventional CSF cytology. CTCs can be found using rare cell capture technology and immune flow cytometry assay with antibodies against epithelial cell adhesion molecule (EpCAM), reporting a sensitivity of 76%-100% and a specificity of 84%-100% for diagnosis of LM[62]. van Bussel et al.[63] reported a sensitivity of 94% (95%CI: 80-99) and a specificity of 100% (95%CI: 91-100), with a cutoff of 0.9 CTC/mL, when using EpCAM immunoflow cytometry. Several studies have shown that the CellSearch technique with immunomagnetic identification and quantification of CSF CTCs improves the ability to diagnose LM from NSCLC, particularly in those patients with conventional acquired negative CSF cytology[64-66]. Moreover, the addition of CSF ctDNA analysis may improve the diagnosis of LM in cases with a low amount of CSF CTCs[67]. Plasma liquid biopsy has been investigated as a surrogate tool for diagnosis of LM from NSCLC. Unfortunately, poor concordance has been reported between plasma and CSF in LM from NSCLC regardless of the type of driver mutation and liquid biopsy technique. Zheng et al.[68] have reported that next-generation sequencing of paired plasma and CSF samples of 11 patients with LM from ALK rearranged NSCLC identified driver mutations in 81.8% of CSF and 45.5% only of plasma. Similarly, Ying et al.[69] have compared CSF and plasma samples of 92 patients with LM from EGFR mutated NSCLC reporting a high mutation rate in CSF (81.5%) with an overall amount of 197 mutations, whereas plasma displayed a lower mutation rate (62.5%) and amount of mutations (68%). Furthermore, a significant discordance of mutation profiles between CSF and plasma has been reported: a further analysis of EGFR showed an activating mutation in 51.4% of CSF and 38.9% of plasma samples with a concordance of 47.7%. Notably, the EGFR T790M resistance mutation was detected in CSF of 2 patients only (2.8%), denoting that mutation occurs more frequently in extracranial sites[67,70,71]. Huang et al.[72] reported similar results when evaluating EGFR status in CSF and plasma (75% vs. 36.4%, respectively) in a cohort of 11 LM cases from EGFR mutated NSCLC, and EGFR T790M mutation was found more frequently in plasma (39%) compared with CSF (13%). Li et al.[73] have found targetable EGFR mutations in CSF of 26/26 patients (100%) and in plasma of 19/26 patients (73.1%). In particular, TP53 loss of heterozygosity (LOH) was identified in CSF of 19/26 patients (73.1%) and in plasma of 2/26 patients only (7.7%), and T790M mutation in 8/26 CSF samples (30.4%) and in 6/26 plasma samples (21.7%). Lastly, Ma et al.[74] reported that 7/13 patients (53.8%), who received TKIs, developed uncommon EGFR mutations in ctDNA of CSF. Interestingly, these uncommon EGFR mutations, including G719A, L861Q, L703P, and G575R mutations, were more frequent in LM (54.5%) than in patients with BM (10%). Overall, CSF liquid biopsy appears to be more sensitive than plasma in detecting druggable mutations in LM. Moreover, CSF has a significant number of specific mutations, such as TP53 LOH, MET amplification, CDKN2A, NTRK1 and CDK4 mutations, that contribute to the tumorigenesis and development of LM from NSCLC[69,75].
Integrated assessment for diagnosis and monitoring of LM from NSCLC
The combination of CSF analysis, including cytology and liquid biopsy, with MRI assessment may improve the ability to diagnose LM. Hyun et al.[58] studied the diagnosis of LM in a cohort of 519 patients with advanced NSCLC; by MRI alone in 35% of patients, by CSF cytology alone in 22%, and by both techniques in 42%[4]. However, the absence of a standardized quantification of LM disease burden represents a challenge. Recently, Nevel et al.[76] have investigated whether MRI disease burden assessment and CSF analysis can be employed to predict survival in LM from NSCLC. For the MRI assessment, the Authors have scored the MRI using 8 predefined anatomic locations, such as cerebrum, ventricles, brainstem, cerebellum, cranial nerves, cervical, thoracic, and lumbosacral spinal cord. One point has been awarded for each affected location regardless of the number of lesions. The presence of ≥ 3 sites of disease was statistically correlated with a risk of death compared with < 3 sites (HR = 1.95; 95%CI: 1.16-3.30; P = 0.01), while anatomic locations of LM were not associated with OS. Regarding CSF analysis, protein level above the upper limit was not correlated with OS, while CSF white blood cell count and low glucose at diagnosis of LM were significantly correlated with OS (P = 0.04 and P < 0.0001, respectively). Importantly, Nevel et al.[76] reported that patients with ≥ 50 CTCs/3 mL had an increased risk of death in comparison with those with < 50 CTCs/3 mL (HR = 3.39; 95%CI: 1.01-11.37; P = 0.048). A further analysis revealed that increased values of ctDNA concentrations (median concentrations of 0.022 ng/µL) were associated with an increased risk of death (HR = 16.33; 95%CI: 0.69-384; P = 0.08). Overall, they suggested that a significant advantage from CTCs count and ctDNA analysis in CSF coupled with a simplified MRI assessment, may help to predict survival. However, only a small number of samples of CSF has been analyzed for CTCs and ctDNA. Thus, it will be important to validate the prognostic value of MRI assessment and advanced CSF techniques in a larger and multicenter cohort of LM from NSCLC.
Treatment options for leptomeningeal metastases from NSCLC
Radiotherapy
Different radiation techniques are investigated in BM, such as stereotactic radiosurgery (SRS), whole-brain radiotherapy (WBRT), intensity modulated radiation therapy (IMRT), or proton beam therapy. Radiotherapy (RT) does not represent the first line treatment in LM for different reasons. First, a retrospective analysis has demonstrated a major impact of systemic chemotherapy and targeted agents in LM control and OS[77]. Moreover, randomized clinical trials evaluating the efficacy and safety of RT in LM have not been conducted thus far. Focal RT, such as involved field or SRS, are considered in patients with local, circumscribed and symptomatic lesions, or in those with CSF flow obstructions due to spinal or intracranial blocks in order to improve the distribution of intra-CSF therapy. Wolf et al.[78] retrospectively analyzed 16 patients with LM from solid tumor (8 NSCLC), treated with SRS, reporting a disease control of 57.1% (partial response in 8 patients) with a median OS of 10 months (6-month and 1-year OS of 60% and 26%, respectively). The Authors suggested that SRS could be added to treat bulky LM in patients also eligible for systemic therapy, including immuno-therapies and targeted therapies, with the aim to prolong OS. WBRT may be considered as palliative treatment in patients with symptomatic extensive nodular or linear LM. Gani et al.[79] reported a median OS of 2 months following WBRT in 27 patients with LM from solid tumors (7 NSCLC). Ozdemir et al.[80] reported a median OS 3.9 months after WBRT in a cohort of 51 LM from NSCLC, and a longer OS (11.3 months) in patients with ECOG 0-1 and without BM. Brower et al.[81] retrospectively analyzed 124 patients with LM from solid tumors (32 NSCLC) and showed a median OS of 9.2 months when WBRT was utilized in conjunction with systemic chemotherapy, with a major benefit in patients with good KPS (KPS ≤ 50: 1.1 months; KPS 60-80: 2.0 months; KPS 90-100: 5.9 months). Notably, Ozdemir and Brower identified some prognostic factors (KPS ≥ 90 and absence of BM) in patients with prolonged OS as compared with historical controls. Craniospinal RT (CSI) is not recommended because of the poor benefit and the significant risk of developing severe adverse effects (myelotoxicity, enteritis and mucositis). Hermann et al.[82] have conducted a retrospective study on 16 patients with LM (5 from NSCLC) treated with CSI alone (6 patients) or in association with intrathecal methotrexate (10 patients), reporting a median OS of 2 months after CSI alone, and 4 months after combined treatment. Interestingly, most of the patients (11/16-68%) experienced significant neurologic improvement (improvement in walking in 7 patients, pain relief in 6 patients, reduction of bladder and bowel incontinence in 3 patients). Devecka et al.[83] reported OS rates in a cohort of 19 patients with LM (5 from NSCLC); a median OS of 7.3 months, 3.3 months and 1.5 months for patients with 0, 1 and 2 risk factors according to the proposed prognostic score (KPS < 70 and the presence of extra-CNS disease), respectively. Recently, Yang et al.[84] have investigated the tolerability of proton CSI in 19 patients with LM(11 from NSCLC) in a phase I trial, reporting a median OS of 8 months (95%CI: 6 to not reached), of whom 4 patients (19%) were disease free ≥ 12 months. Two patients only reported grade 4 lymphopenia, grade 4 thrombocytopenia, and grade 3 fatigue.
The US National Comprehensive Cancer Network (NCCN) 2020 guidelines for management of LM recommend focal RT in association with intrathecal chemotherapy in patients with favorable prognostic factors (KPS ≥ 60, mild neurologic deficits, stable systemic disease, available therapeutic options for systemic disease). For patients who do not meet these criteria, focal RT to symptomatic lesions or best supportive care, are the suggested options[85][Figure 3]. Lastly, the use of IMRT or proton therapy for treatment of LM should not be considered as usual therapy.
Intrathecal therapy
Intrathecal therapy can be administered by lumbar puncture or an intraventricular route. Prolonged survival has been demonstrated using intraventricular route (e.g., Ommaya reservoir)[86], but the management of the device may be difficult, and careful handling is required to avoid obstruction[87]. Furthermore, some complications may occur following intrathecal therapy, including aseptic or chemical meningitis, arachnoiditis, and delayed leukoencephalopathy with seizures[87]. In general, intrathecal compounds can only penetrate the tissue for 2-3 millimeters, thus it is preferred in patients with linear leptomeningeal lesions and non-bulky disease. Three drugs are commonly used: methotrexate (MTX), liposomal cytarabine (Ara-C) and thioTEPA. Three clinical trials have investigated the efficacy of intrathecal therapy in LM from solid tumors, including NSCLC, reporting OS between 1.7-11 months [Table 3]. Younger age (< 55 years), absence of systemic metastases or cranial nerve involvement, normal value of CSF glucose and proteins are considered favorable prognostic factors[91]. Lin et al.[92] reported a case of LM from NSCLC, who received intrathecal chemotherapy with pemetrexed via Ommaya reservoir. The local treatment led to an improvement of the quality of life, as well as the clearing of CSF cytology and stable LM disease for 17 months. Wu et al.[93] have conducted a pooled analysis, that evaluated intrathecal chemotherapy in NSCLC patients. Overall, 4 prospective studies and 5 retrospective studies were included. Thirty-seven patients were treated with intrathecal chemotherapy alone, and 552 patients received multiple interventions (intrathecal chemotherapy, WBRT, EGFR TKIs, traditional chemotherapy, and supportive care). The clinical response of the patients receiving intrathecal chemotherapy alone ranged between 71% to 79% with a median OS longer (7.5 months) than that of patients who received combined treatments (3.0-5.0 months). Overall, the efficacy of intrathecal therapy is modest, and careful evaluation of clinical factors helps clinicians to identify the subgroups of patients who may benefit.
Randomised clinical trials on intrathecal chemotherapy in LM from NSCLC
| Study | No. of patients | Intrathecal therapy | Results |
|---|---|---|---|
| Grossman et al.[88], 1993 | 59 (15 NSCLC) | Arm 1: MTX 10 mg twice weekly
Arm 2: thioTEPA 10 mg twice weekly | Median OS:
MTX: 3.9 months thioTEPA: 3.5 months No patients have neurological improvement, and 75% had neurologic improvement |
| Hitchins et al.[89], 1987 | 44 (13 NSCLC) | Arm 1: MTX 15 mg twice weekly
Arm 2: MTX 15 mg twice weekly plus Ara-C 50 mg/m2 every 2 weeks | Median OS:
MTX: 4.5 months MTX/Ara-C: 1.7 months Radiological response to MTX was superior to combined MTX/Ara-C (61% vs. 45%), but not statistically significant |
| Glantz et al.[90], 1999 | 61 (6 NSCLC) | Arm 1: Ara-C 50 mg/m2 every 2 weeks
Arm 2: MTX 10 mg twice weekly | Median OS:
MTX: 3.3 months Ara-C: 11 months Radiological response to Ara-C was superior to combined MTX (26% vs. 20%), but not statistically significant |
Systemic chemotherapy for LM from NSCLC
A standard treatment is not validated thus far, but platinum based-chemotherapy with or without RT is recommended in patients with LM from NSCLC, who have no druggable mutations or programmed death-ligand 1 (PD-L1) tumor proportion score < 50[3]. The median OS in patients with good prognostic factors is approximately 11.5 months following traditional chemotherapy[94]. Bevacizumab has been investigated in LM after failure of first-generation EGFR TKIs with clinical and radiological response in 2 patients with LM from EGFR mutated NSCLC, who progressed after first-line treatment with erlotinib[95].
Targeted agents
Approximately 20%-25% of patients with NSCLC have oncogene driver mutations: the most frequent is the EGFR (10%-15%) followed by the ALK rearrangement (3%-5%), while PD-L1 expression ranges from 21.9% to 32.9%. Less frequent mutations are KRAS, MET, ROS1, BRAF, and HER2. Targeting some of these mutations has shown a significant advantage in BM from NSCLC. Now, the activity of targeted therapy in LM is under investigation in clinical trials.
Role of EGFR tyrosine kinase inhibitors in LM from NSCLC
Two retrospective studies have shown that LM is more common in patients with EGFR mutations (9.4%) than in EGFR wild-type (1.7%)[96,97]. Different studies have investigated the efficacy of first- and second-generation EGFR TKIs in LM [Table 4]. A low CSF level (1%-3%) of the first-generation EGFR TKIs has been found, suggesting an inability to adequately penetrate the BBB. Therefore, higher or “pulsatile” doses of either gefitinib or erlotinib have been administered in order to achieve adequate therapeutic concentrations, reporting a median OS ranging from 3 to 12 months[31,98-101]. The second-generation EGFR TKI, afatinib, has shown some activity in 11 LM patients with uncommon EGFR mutation (Gly719X) that were pre-treated with erlotinib or gefitinib. Twenty-seven percent of patients achieved significant radiological response, with a median PFS and OS of 2 months and 3.8 months, respectively[15]. The third-generation EGFR TKI osimertinib has demonstrated remarkable activity to control systemic and CNS disease[102]. In light of that, osimertinib represents the first-line treatment, regardless of T790M mutation, and it is considered the preferred initial therapy when feasible[103]. The increased ability to cross the BBB makes osimertinib an attractive compound to be investigated in LM. Nanjo et al.[17] have investigated the standard dose of osimertinib (80 mg) in LM after failure of first- and second-generation TKIs. The Authors reported a CSF clearance in 2 patients out of 5 with a definitive diagnosis of LM, and a median PFS of 7.2 months. Notably, osimertinib was active on CSF malignant cells either with T790M or Leu858Arg mutations[17]. The phase I BLOOM study demonstrated good activity of high-dose osimertinib (160 mg/day) in 41 patients with LM who were heavily pretreated with TKIs. The intracranial objective response rate (ORR) was 62% (95%CI: 45-78), the median OS was 11.0 months (95%CI: 8.0-18.0 months), and a CSF tumor cell clearance was confirmed in 11 patients (28%)[104]. Saboundji et al.[105] retrospectively analyzed a cohort of 20 patients treated with osimertinib: all patients (100%) displayed neurological improvement, and 5 patients (20%) showed prompt radiological response within 15 days from the start of treatment. For this cohort, median PFS and OS were of 17.2 and 18 months, respectively. Similar results were reported by Ahn et al.[106], retrospectively analyzing 22 patients with LM from EGFR T790M mutated NSCLC treated with osimertinib 80 mg/day: ORR was 55% (95%CI: 32-76), and median OS was 18.8 months (95%CI: 6.3-NC). Park et al.[107] also reported an intracranial ORR of 55.0%, median PFS of 7.6 months (95%CI: 5.0-16.6), and median OS of 16.9 months (95%CI: 7.9-not reached) in a phase II trial cohort of 40 patients with LM treated with osimertinib 160 mg daily. Interestingly, osimertinib is not only active against T790M mutation, but may also target uncommon mutations, such as S768I mutation[108].
Studies on EGFR TKIs in patients with LM from non-small-cell lung cancer
| Study | No. of patients | Treatment | Outcomes |
|---|---|---|---|
| Grommes et al.[31], 2011
Retrospective | 9 | Pulsatile high-dose erlotinib (1500 mg weekly) | Radiological response in 6/9 patients (66.7%)
Median OS: 12 months |
| Lee et al.[98], 2013
Retrospective | 25 | Arm 1: gefitinib 250 mg/day
Arm 2: erlotinib 150 mg/day | CSF citology in 10/25 patients (40%)
Erlotinib led to CSF citology conversion in 64.3% of patients, while 9.1% only following gefitinib |
| Yang et al.[99], 2015
Retrospective | 6 | Pemetrexed 500 mg/m2 day 1; cisplatin 30 mg day 1-2; erlotinib 150 mg day 3-21 | Response rate: CR 1/6 (16.6%); PR 2/6 (33.3%); SD 2/6 (33.3%)
Median OS: 9 months |
| Kawamura et al.[100], 2015
Retrospective | 35 | Arm 1: high-dose erlotinib (200-600 mg/day every 2-4 days)
Arm 2: standard dose erlotinib (150 mg/day) | High-dose erlotinib: radiological response in 3/10 patients (30%), neurological improvement in 6/12 patients (50%)
Median OS: - high-dose group: 6.2 months Standard dose group: 5.9 months |
| Jackman et al.[101], 2015
Phase I | 7 | 2 weeks of high-dose of gefitinib (750-1000 mg/day) and 2 weeks of 500 mg/day | Median OS: 3.5 months
Median PFS: 2.3 months CSF citology clearance in 1/7 patients (14.3%) Neurological improvement in 4/7 patients (57.1%) |
| Liao et al.[102], 2015
Retrospective | 75 | Arm A: Gefitinib + CT
Arm B: Erlotinib + CT Arm C: Afatinib + CT Regimen details not available | The association of TKI plus chemotherapy is correlated with prolonged survival in both univariate and multivariate analysis |
| Tamiya et al.[15], 2017
Prospective | 11 | Afatinib 40 mg/m2 daily | Median CSF penetration: 1.65%
Median CSF concentration: 1.4 ng/mL (2.9 nmol/L) Radiological response: 27.3% Median PFS: 2 months Median OS: 3.8 months |
| Yang et al.[16], 2017
Phase I | 32 | Osimertinib 160 mg daily | 20/23 patients (86.9%) had neurological improvement
23/32 (72%) had radiological response |
| Nanjo et al.[17], 2017
Prospective | 13 (3 definitive LM and 8 possible LM) | Osimertinib 80 mg daily | CSF penetration: 2.5%
Median PFS: 7.2 months |
| Yang et al.[104], 2020
Prospective | 41 | Osimertinib 160 mg daily | ORR 62%
Median OS 15.2 months |
| Saboundji et al.[105], 2018
Retrospective | 20 | Osimertinib 80 mg daily | 100% of patients experienced neurological improvement
Median PFS: 17.2 months Median OS: 18 months |
| Ahn et al.[106], 2020
Retrospective | 22 | Osimertinib 80 mg daily | ORR 55%
Median OS 18.8 months |
| Park et al.[107], 2020
Phase 2 | 40 | Osimertinib 160 mg daily | ORR 55%
Median PFS 7.6 months Median OS 16.9 months |
| Ahn et al.[109], 2016
Prospective | 29 (4 with LM) | AZD3759 | 3/4 patients (75%) had a significant reduction of EGFR expression
1/4 patients (25%) had a CSF conversion in two consecutive samples |
| Cho et al.[110], 2017
Prospective | 18 | Arm 1: AZD3759 200 mg daily
Arm 2: AZD3759 300 mg daily | 5/18 patients (27.8%) had a radiological response, while 9/18 patients (50%) a stable disease |
| Xu et al.[112], 2020
Prospective | 3 | Erlotinib (150 mg/day) plus nimotuzumab (200 mg/m2) weekly | Rapid clinical response within 6-8 weeks from the start of treatment
2/3 patients reported a radiological response |
AZD3759 is a novel compound with excellent BBB penetration, which is active against EGFR mutations, with the exception of T790M mutation. The efficacy and tolerability of AZD3759 have been investigated in 29 patients in a phase I trial. Of the four patients with LM who were enrolled, 3 displayed a significant reduction of EGFR expression on the cell surface, and one patient had a CSF conversion in two consecutive samples[109]. Cho et al.[110] have investigated the efficacy of AZD3759 at two different doses (200 mg or 300 mg) reporting in 5/18 patients (27.8%) a radiological response, and in 9/18 patients (50%) a stable disease. A new anti-EGFR monoclonal antibody, nimotuzumab, has demonstrated some activity in BM from NSCLC[111]. Xu et al.[112] have used nimotuzumab in association with erlotinib in 3 patients with advanced LM reporting clinical improvement within 6-8 months from the start of treatment and a radiological response in 2/3 patients.
Role of ALK inhibitors in LM from NSCLC
LM in ALK rearranged NSCLC tends to occur in approximately 5% of patients as a late complication after a median time of 9 months from the diagnosis of the systemic tumor[6]. Although the benefit from ALK inhibitors has been established in BM, data regarding the activity in LM are limited to case-reports. The first-generation ALK, ROS1, and MET inhibitor crizotinib has demonstrated remarkable CNS disease control rate (55% and 65% at 12 and 24 weeks, respectively) in patients with BM[23,113]. Three case reports described the activity from the association of crizotinib and WBRT or intrathecal methotrexate in LM, reporting a PFS of 6-10 months [Table 5][19,114]. The second-generation ALK/ROS1 inhibitor ceritinib displayed significant systemic and intracranial activity in patients with ALK rearrangement who were pretreated with crizotinib[115,116]. Ceritinib has been also reported to be active in LM in association with either traditional chemotherapy or WBRT in patients who progressed after failure of crizotinib, with a median PFS of 5-18 months[117,118]. The phase II ASCEND 7 trial has investigated ceritinib in BM and LM from ALK rearranged NSCLC. Forty-two patients previously treated with radiotherapy and an ALK inhibitor were assigned to arm 1; 40 patients with prior ALK inhibitor alone were assigned to arm 2; 12 patients with prior radiotherapy alone were assigned to arm 3; and 44 patients not previously treated with radiotherapy or an ALK inhibitor were assigned to arm 4. Evaluation of the intracranial response was done in 28, 29, 7, and 33 patients in the respective arms having measurable BM at baseline, and showed an intracranial objective response rate (ORR) of 39.3%, 27.6%, 28.6%, and 51.5% in arms 1, 2, 3, and 4, respectively. Unfortunately, the trial reported the intracranial ORR for BM only, without any details regarding LM response[119]. Alectinib, which is a second-generation ALK/RET inhibitor, has been approved either after crizotinib and as a first-line treatment for ALK rearranged NSCLC[120,121]. Different case series on LM have reported significant and durable radiological responses with both standard (600 mg twice daily) and increased dose (900 mg twice daily)[122-124]. Moreover, the J-ALEX trial has compared the efficacy of alectinib or crizotinib as first line-treatment in BM and asymptomatic LM; however, results for the last subgroup have not been reported[120]. Brigatinib, a potent ALK/ROS/EGFR inhibitor, had an impressive intracranial ORR of 53%-67% with a median PFS > 12 months when used in BM[125]. Gaye et al.[126] reported a case of LM pretreated with first- and second-generation ALK inhibitors, and achieved a median PFS of 14 months following brigatinib. Overall, the efficacy of brigatinib in LM needs to be further investigated, and the phase 3 ALTA-1L trial includes patients with any CNS recurrence. Preliminarly, the intracranial ORR was 67% with a median PFS of 11 months, but response of LM was not analyzed separately from that of BM[127]. Lorlatinib, which is an ALK/ROS1 inhibitor with an excellent BBB penetration, led to an intracranial ORR of 44% in ALK rearranged NSCLC patients heavily pretreated with ALK inhibitors[23]. Moreover, a recent case report described a significant and long-lasting response of a spinal LM, achieving a PFS of 12 months from the start of treatment[128].
Studies on ALK inhibitors in patients with LM from non-small-cell lung cancer
| Study | No. of patients | Treatment | Outcomes |
|---|---|---|---|
| Costa et al.[28], 2011 | 1 | WBRT plus crizotinib 250 mg twice daily | PFS:9 months |
| Ahn et al.[114], 2012 | 2 | Intrathecal MTX plus crizotinib 250 mg twice daily | PFS 5 and 10 months, respectively |
| Arrondeau et al.[117], 2014 | 1 | Ceritinib 750 mg daily | PFS: 5.5 months |
| Dudnik et al.[118], 2015 | 3 | WBRT plus ceritinib 500 mg/daily | PFS - Patients 1: 18 months
Patient 2 and 3: 7 months |
| Gainor et al.[122], 2015 | 4 | Alectinib 600 mg twice daily | Radiological and neurological improvement in 4/4 patients (75%) |
| Ou et al.[123], 2015 | 1 | Alectinib 600-750 mg twice daily | Long-lasting complete response (15 months) |
| Gainor et al.[124] 2016 | 2 | Alectinib 900 mg twice daily | Radiological and neurological improvement for 3.5 and 6 months, respectively |
| Gaye et al.[126], 2019 | 1 | Brigatinib 180 mg once daily with a 7-day lead-in period at 90 mg | PFS 14 months |
| Pellerino et al.[128], 2019 | 1 | Lorlatinib 100 mg once daily | PFS 12 months
Complete radiological response |
Immunotherapy for LM from NSCLC
Inhibitors of the programmed death-1 (PD-1)/PD-ligand 1 pathways, such nivolumab and pembrolizumab, have shown some efficacy in patients with advanced NSCLC patients with pretreated BM[129-132]. The PD-L1 expression and tumor-infiltrating lymphocytes have been suggested to be predictive factors for response to immune checkpoint inhibitors (ICIs)[133], but their expression in LM remain unknown. Gion et al.[134] described neurological improvement lasting 7 months in patients with LM treated with nivolumab, and Dudnik et al.[135] reported 1 partial response and 1 stable disease lasting > 21 and 10 weeks, respectively, in 2 patients with LM after a treatment with nivolumab. A prospective evaluation of 19 patients with LM from NSCLC, who were treated with ICIs (13 with nivolumab and 6 with pembrolizumab), showed a median PFS of 3.7 months, and a 6- and 12-months OS of 36.8 and 21.1%, respectively[136]. Brastianos et al.[137] have investigated the efficacy of pembrolizumab in a phase 2 trial of 20 patients with LM (2 from NSCLC). The study met the primary endpoint, as 12/20 (60%, 90%CI: 0.39-0.78) patients were alive at 3 months after enrollment. The activity of pembrolizumab is now being investigated in a phase 2 study focused on LM from NSCLC (NCT03091478). The Table 6 summarizes the ongoing clinical trials on LM from NSCLC.
Ongoing clinical trials on LM from NSCLC
| Study | No. of patients | Treatment | Outcomes |
|---|---|---|---|
| NCT04356118
Phase 4 | 30 | Recombinant human endostatin 7.5 mg/m2/day once a day for 2 weeks and 1 week off plus intrathecal MTX plus targeted therapy (EGFR TKIs or ALK inhibitors) | Primary:
OS Neurological PFS Adverse events Secondary: ORR |
| NCT04343573
Phase 2 | 100 | Arm 1: proton CSI (30 Gy/30 fr) plus standard of care for LM per physician choice
Arm 2: proton CSI (30 Gy/10 fr) alone | Primary:
CNS PFS Secondary: OS |
| NCT04356222
Phase 4 | 30 | Durvalumab 10 mg/kg every 2 weeks plus intrathecal MTX | Primary:
OS Neurological PFS Adverse events Secondary: ORR |
| NCT04192981
Phase 1 | 36 | WBRT (30 Gy/10 fr) plus GDC-0084 in 3+3 dose-escalation cohort: 45, 60, 75 mg daily, with a potential dose de-escalation cohort to 30 mg | Primary:
MTD Secondary: Local recurrence rate |
| NCT04425681
Phase 2 | 20 | Osimertinib 80 mg daily plus bevacizumab 7.5 mg/kg every 3 weeks | Primary:
PFS ORR Secondary: Adverse events |
| NCT04148898
Phase 2 | 80 | Arm 1: Osimertinib 80 mg daily alone
Arm 2: Osimertinib 80 mg daily plus bevacizumab 7.5 mg/kg every 3 weeks | Primary:
PFS ORR Secondary: OS Adverse events |
| NCT03719768
Phase 1b | 23 | Avelumab 800 mg iv every 2 weeks plus WBRT (30 Gr/10 fr) | Primary:
Safety and dose limiting toxicity Secondary: ORR Number of T cells in CSF Activation status of T cells in CSF |
| NCT03091478
Phase 2 | 13 | Pembrolizumab 200 mg every 3 weeks | Primary:
ORR |
Steroids in the management of LM
Steroids are frequently used in daily clinical practice for the treatment of neurological symptoms from LM. Considering the long half-life that allows the administration in a single daily dose, dexamethasone is the most used steroid. The main advantage is represented by the significant glucocorticoid potency, associated with the virtual absence of mineralocorticoid effects, resulting in a decreased risk of electrolyte imbalances compared with other steroids. The main effects are to decrease the permeability of the BBB and limit the extravasation of fluid[138,139], and antiemetic properties by reducing the cellular 5-HT3 receptor expression on the medulla oblongata[140,141]. In general, steroids are employed to reduce meningeal irritation and radicular pain from LM or chemical meningitis following intrathecal chemotherapy[57]. Studies on dosing and tapering of dexamethasone in LM have not been performed thus far, therefore the dose should be tailored to each patient’s individual needs. In general, the lowest dose of steroids should be used for the shortest time possible to limit adverse events, such as arterial hypertension, increased risk of fungal infections, osteoporosis, diabetes, myopathy, and psychiatric effects (e.g., insomnia, emotional lability, hypomanic and manic episodes)[142].
Prognostic factors
In general, there are two models used for predicting outcomes in patients with LM. The first is based on the general condition of patients detected by Karnofsky performance score (KPS), neurologic symptoms, and presence of extracranial metastases. In this regard, the US National Comprehensive Cancer Network (NCCN) guidelines (version 2, 2020) for patients with LM stratifies patients as “good risk” or “poor risk”[85]. Good risk patients have KPS ≥ 60, mild neurologic deficits, no bulky disease, stable systemic disease, and available therapeutic options for systemic disease, resulting in a prolonged survival compared with “poor risk patients. The MRI presentation of LM has been suggested to impact the survival: in particular, a diffuse linear enhancement LM has been correlated with a prolonged OS compared with nodular LM from NSCLC[4,57]. Those 2 models may not be reliable to predict the prognosis of LM from NSCLC without an integration of molecular markers, especially in patients with druggable mutations. Some authors have suggested a prognostic assessment integrated with molecular alterations (molGPA) to predict the outcome of LM patients from NSCLC. In particular, 301 patients with LM from NSCLC were scored using the molGPA and classified them into 3 prognostic groups of high, intermediate and low risk (molGPA score of 0, 0.5-1.0 and 1.5-2.0, respectively). The median OS of high, intermediate and low risk LM patients were 0.3, 3.5 and 15.9 months, respectively (P < 0.001). Moreover, EGFR/ALK positivity, KPS ≥ 60, and absence of extracranial metastases are independent predictive factors for better OS[143].
Conclusion
The leptomeningeal space remains a sanctuary site, with limited penetration of drugs. Recent advances in diagnosis and treatment have been made, but several issues are still unaddressed. A standardized MRI assessment for evaluating LM at diagnosis and during follow needs to be validated in prospective cohorts. Similarly, CSF liquid biopsy could be a useful tool for diagnosis and monitoring of LM, especially in those patients with equivocal MRI findings, but sensitivity and specificity of different liquid biopsy techniques have to be compared, and cut-off values should be identified. The third-generation EGFR inhibitor osimertinib has demonstrated significant CNS penetration and survival advantage in EGFR-mutated LM compared with standard therapies, such as RT, traditional chemotherapy, first- and second-generation TKIs. The combination of third-generation TKIs with RT or traditional chemotherapy could provide an additional advantage in terms of quality of life and disease control. Several case-reports have reported some efficacy of ALK inhibitors in LM, but clinical trials should be designed to confirm this benefit. For those patients who do not have druggable mutations, ICIs could represent a therapeutic option with acceptable tolerability, but clinical trials focused on LM from NSCLC are lacking and represent a research focus for the future.
Declarations
Authors’ contributionsConceptualization, writing original draft and preparation: Pellerino A, Soffietti R, Rudà R
Data collection and curation: Pellerino A, Internò V, Muscolino E, Mo F, Bruno F, Pronello E, Franchino F, Soffietti R, Rudà R
Writing review and editing: Pellerino A, Internò V, Muscolino E, Mo F, Bruno F, Pronello E, Franchino F
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2020.
REFERENCES
1. Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: leptomeningeal metastases in solid tumors. Surg Neurol Int 2013;4:S265-88.
2. Le Rhun E, Galanis E. Leptomeningeal metastases of solid cancer. Curr Opin Neurol 2016;29:797-805.
3. Pan Z, Yang G, He H, Yuan T, Wang Y, et al. Leptomeningeal metastasis from solid tumors: clinical features and its diagnostic implication,. Sci Rep 2018;8:10445.
4. Remon J, Le Rhun E, Besse B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: A continuing challenge in the personalized treatment era. Cancer Treat Rev 2017;53:128-37.
5. Lee Y, Han JY, Kim HT, Yun T, Lee GK, et al. Impact of EGFR tyrosine kinase inhibitors versus chemotherapy on the development of leptomeningeal metastasis in never smokers with advanced adenocarcinoma of the lung. J Neurooncol 2013;115:95-101.
6. Gainor JF, Ou SH, Logan J, Borges LF, Shaw AT. The central nervous system as a sanctuary site in ALK-positive non-small-cell lung cancer. J Thorac Oncol 2013;8:1570-3.
7. van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, et al. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat 2015;19:1-12.
8. Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med 2013;19:1584-96.
9. Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab 2012;32:1959-72.
10. Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis 2010;37:13-25.
11. Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx 2005;2:3-14.
12. Liu Y, Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell 2016;30:668-81.
13. Sprowls SA, Arsiwala TA, Bumgarner JR, Shah N, Lateef SS, et al. Improving CNS delivery to brain metastases by blood-tumor barrier disruption. Trends Cancer 2019;5:495-505.
14. Ota K, Shiraishi Y, Harada T, Himeji D, Kitazaki T, et al. Phase II study of erlotinib in advanced non-small cell lung cancer patients with leptomeningeal metastasis (LOGIK1101). J Thorac Oncol 2017;12:S271-2.
15. Tamiya A, Tamiya M, Nishihara T, Shiroyama T, Nakao K, et al. Efficacy and cerebrospinal fluid concentration of afatinib in NSCLC patients with EGFR mutation developing leptomeningeal carcinomatosis. J Thorac Oncol 2017;12:S273.
16. Yang JC, Cho BC, Kim D, Kim SW, Lee JS, et al. Osimertinib for patients (pts) with leptomeningeal metastases (LM) from EGFR-mutant non-small cell lung cancer (NSCLC): updated results from the BLOOM study. Proc Am Soc Clin Oncol 2017;35:2020. (abstr)
17. Nanjo S, Hata A, Okuda C, Kaji R, Okada H, et al. Standard-dose osimertinib for refractory leptomeningeal metastases in T790M-positive EGFR-mutant non-small cell lung. Br J Cancer 2018;118:32-7.
18. Ahn M, Kim D, Kim TM, Lin CC, Ratnayake J, et al. Phase I study of AZD3759, a CNS penetrable EGFR inhibitor, for the treatment of non-small-cell lung cancer (NSCLC) with brain metastasis (BM) and leptomeningeal metastasis (LM). Proc Am Soc Clin Oncol 2016;34:9003. (abstr)
19. Costa DB, Kobayashi S, Pandya SS, Yeo WL, Shen Z, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;29:e443-5.
20. Zhang I, Zaorsky NG, Palmer JD, Mehra R, Lu B. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol 2015;16:e510-21.
21. Sakamoto H, Tsukaguchi T, Hiroshima S, Kodama T, Kobayashi T, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell 2011;19:679-90.
22. Kodama T, Hasegawa M, Takanashi K, Sakurai Y, Kondoh O, et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol 2014;74:1023-8.
23. Solomon B, Bauer TM, Felip E, Besse B, Philip L, et al. Safety and efficacy of lorlatinib (PF-06463922) from the dose-escalation component of a study in patients with advanced ALK+ or ROS1+ non-small cell lung cancer (NSCLC). Proc Am Soc Clin Oncol 2016;34:9009. (abstr)
24. Demeule M, Régina A, Jodoin J, Laplante A, Dagenais C, et al. Drug transport to the brain: key roles for the efflux pump P-glycoprotein in the blood-brain barrier. Vascul Pharmacol 2002;38:339-48.
25. Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol 2018;19:e43-55.
26. Chamberlain MC, Baik CS, Gadi VK, Bhatia S, Chow LQM. Systemic therapy of brain metastases: non-small cell lung cancer, breast cancer, and melanoma. Neuro Oncol 2017;19:1-24.
27. Pardridge WM. CSF, blood-brain barrier, and brain drug delivery. Expert Opin Drug Deliv 2016;13:963-75.
28. Kummar S, Rubinstein L, Kinders R, Parchment RE, Gutierrez ME, et al. Phase 0 clinical trials: conceptions and misconceptions. Cancer J 2008;14:133-7.
29. Arbour KC, Kris MG, Riely GJ, Ni A, Beal K, et al. Twice weekly pulse and daily continuous-dose erlotinib as initial treatment for patients with epidermal growth factor receptor-mutant lung cancers and brain metastases. Cancer 2018;124:105-9.
30. Clarke JL, Pao W, Wu N, Miller VA, Lassman AB. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol 2010;99:283-6.
31. Grommes C, Oxnard GR, Kris MG, Miller VA, Pao W, et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol 2011;13:1364-9.
32. Wang S, Chen J, Xie Z, Xia L, Luo W, et al. Pulsatile crizotinib treatment for brain metastasis in a patient with non-small-cell lung cancer. J Clin Pharm Ther 2017;42:627-30.
33. Pellerino A, Bertero L, Rudà R, Soffietti R. Neoplastic meningitis in solid tumors: from diagnosis to personalized treatments. Ther Adv Neurol Disord 2018;11:1756286418759618.
34. Roelz R, Reinacher P, Jabbarli R, Kraeutle R, Hippchen B, et al. Surgical ventricular entry is a key risk factor for leptomeningeal metastasis of high grade gliomas. Sci Rep 2015;5:17758.
35. Ahn JH, Lee SH, Kim S, Shin SH, Gwak HS, et al. Risk for leptomeningeal seeding after resection for brain metastases: implication of tumor location with mode of resection. J Neurosurg 2012;116:984-93.
36. Ojerholm E, Lee JY, Thawani JP, Miller D, Alonso-Basanta M, et al. Stereotactic radiosurgery to the resection bed for intracranial metastases and risk of leptomeningeal carcinomatosis. J Neurosurg 2014;121:75-83.
37. Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 2017;18:1040-8.
38. Foreman PM, Jackson BE, Singh KP, Romeo AK, Guthrie B, et al. Postoperative radiosurgery for the treatment of metastatic brain tumor: Evaluation of local failure and leptomeningeal disease. J Clin Neurosci 2018;49:48-55.
39. Boire A, Zou Y, Shieh J, Macalinao DG, Pentsova E, et al. Complement component 3 adapts the cerebrospinal fluid for leptomeningeal metastasis. Cell 2017;168:1101-13.e13.
40. Conrad C, Dorzweiler K, Miller MA, Lauffenburger DA, Strik H, et al. Profiling of metalloprotease activities in cerebrospinal fluids of patients with neoplastic meningitis. Fluid Barriers CNS 2017;14:22.
41. Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov 2015;5:1164-77.
42. Shih DJH, Nayyar N, Bihun I, Dagogo-Jack I, Gill CM, et al. Genomic characterization of human brain metastases identifies drivers of metastatic lung adenocarcinoma. Nat Genet 2020;52:371-7.
43. Nanjo S, Arai S, Wang W, Takeuchi S, Yamada T, et al. MET copy number gain is associated with gefitinib resistance in leptomeningeal carcinomatosis of EGFR-mutant lung cancer cells. Mol Cancer Ther 2017;16:506-15.
44. Balak MN, Gong Y, Riely GJ, Somwar R, Li AR, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res 2006;12:6494-501.
45. Ohara S, Ushijima T, Gunji I, Tanai C, Tanaka Y, et al. Brain metastasis effectively treated with erlotinib following the acquisition of resistance to gefitinib: a case report. J Med Case Rep 2014;8:64.
46. Fan Y, Hu M, Zhu X, Wang M, Xu Y, et al. Exploration of the underlying mechanisms of leptomeningeal metastasis in NSCLC patients through NGS of cerebrospinal fluid. J Thorac Oncol 2017;12:S271.
47. Jiang BY, Li Y, Chuai S, et al. NGS to reveal heterogeneity between cerebrospinal fluid and plasma ctDNA among non-small cell lung cancer patients with leptomeningeal carcinomatosis. Proc Am Soc Clin Oncol 2017;35:9022.
48. Oxnard GR, Arcila ME, Sima CS, Riely GJ, Chmielecki J, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumor harboring the T790M mutation. Clin Cancer Res 2011;17:1616-22.
49. Lombardi G, Zustovich F, Farina P, Puppa AD, Manara R, et al. Neoplastic meningitis from solid tumors: new diagnostic and therapeutic approaches. Oncologist 2011;16:1175-88.
50. Nayak L, DeAngelis LM, Brandes AA, Peereboom DM, Galanis E, et al. The neurologic assessment in neuro-oncology (NANO) scale: a tool to assess neurologic function for integration into the response assessment in neuro-oncology (RANO) criteria. Neuro Oncol 2017;19:625-35.
51. Mason W. NANO, a practical scale for neurologic assessments in patients with brain tumors? Neuro Oncol 2017;19:603-4.
52. Chamberlain M, Junck L, Brandsma D, Soffietti R, Rudà R, et al. Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol 2017;19:484-92.
53. Chamberlain M, Soffietti R, Raizer J, Rudà R, Brandsma D, et al. Leptomeningeal metastasis: a response assessment in neuro-oncology critical review of endpoints and response criteria of published randomized clinical trials. Neuro Oncol 2014;16:1176-85.
54. Singh SK, Leeds NE, Ginsber LE. MR imaging of leptomeningeal metastases: comparison of three sequences. AJNR Am J Neuroradiol 2002;23:817-21.
55. Chamberlain MC. Comprehensive neuraxis imaging in leptomeningeal metastasis: a retrospective case series. CNS Oncol 2013;2:121-8.
56. Le Rhun E, Devos P, Boulanger T, Smits M, Brandsma D, et al. The RANO leptomeningeal metastasis group proposal to assess response to treatment: lack of feasibility and clinical utility and a revised proposal. Neuro Oncol 2019;21:648-58.
57. Le Rhun E, Weller M, Brandsma D, Van den B, de Azambuja H, et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol 2017;28:iv84-99.
58. Hyun JW, Jeong IH, Joung A, Cho HJ, Kim SH, et al. Leptomeningeal metastasis: clinical experience of 519 cases. Eur J Cancer 2016;56:107-14.
59. Freilich RJ, Krol G, DeAngelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol 1995;38:51-7.
60. Komori T, Delbeke D. Leptomeningeal carcinomatosis and intramedullary spinal cord metastases from lung cancer: detection with FDG positron emission tomography. Clin Nucl Med 2001;26:905-7.
61. Lee SJ, Lee JI, Nam DH, Ahn YC, Han JH, et al. Leptomeningeal carcinomatosis in non-small-cell lung cancer patients: impact on survival and correlated prognostic factors. J Thorac Oncol 2013;8:185-91.
62. Boire A, Brandsma D, Brastianos PK, Le Rhun E, Ahluwalia M, et al. Liquid biopsy in central nervous system metastases: a RANO review and proposals for clinical applications. Neuro Oncol 2019;21:571-84.
63. van Bussel MTJ, Pluim D, Milojkovic Kerklaan B, Bol M, Sikorska K, et al. Circulating epithelial tumor cell analysis in CSF in patients with leptomeningeal metastases. Neurology 2020;94:e521-8.
64. Jiang BY, Li YS, Guo WB, Zhang XC, Chen ZH, et al. Detection of driver and resistance mutations in leptomeningeal metastases of NSCLC by next-generation sequencing of cerebrospinal fluid circulating tumor cells. Clin Cancer Res 2017;23:5480-8.
65. Nayak L, Fleisher M, Gonzalez-Espinoza R, Lin O, Panageas K, et al. Rare cell capture technology for the diagnosis of leptomeningeal metastasis in solid tumors. Neurology 2013;80:1598-605.
66. Tu Q, Wu X, Le Rhun E, Blonski M, Wittwer B, et al. CellSearch technology applied to the detection and quantification of tumor cells in CSF of patients with lung cancer leptomeningeal metastasis. Lung Cancer 2015;90:352-7.
67. Sasaki S, Yoshioka Y, Ko R, Katsura Y, Namba Y, et al. Diagnostic significance of cerebrospinal fluid EGFR mutation analysis for leptomeningeal metastasis in non-small-cell lung cancer patients harboring an active EGFR mutation following gefinitib therapy failure. Respir Investig 2016;54:14-9.
68. Zheng MM, Li YS, Jiang BY, Tu HY, Tang WF, et al. Clinical utility of cerebrospinal fluid cell-free DNA as liquid biopsy for leptomeningeal metastases in ALK-rearranged NSCLC. J Thorac Oncol 2019;14:924-32.
69. Ying S, Ke H, Ding Y, Liu Y, Tang X, et al. Unique genomic profiles obtained from cerebrospinal fluid cell-free DNA of non small cell lung cancer patients with leptomeningeal metastases. Cancer Biol Ther 2019;20:562-70.
70. Hata A, Katakami N, Yoshioka H, Takeshita J, Tanaka K, et al. Rebiopsy of non-small cell lung cancer patients with acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor: comparison between T790M mutation-positive and mutation-negativee populations. Cancer 2013;119:4325-32.
71. Zhao J, Ye X, Xu Y, Chen MJ, Zhong W, et al. EGFR mutation status of paired cerebrospinal fluid and plasma samples in EGFR mutant non-small cell lung cancer with leptomeningeal metastases. Cancer Chemother Pharmacol 2016;78:1305-10.
72. Huang R, Ge M, Zhou X, Ji X, Liao L, et al. Epidermal growth factor receptor mutation detection in cerebrospinal fluid of lung adenocarcinoma patients with leptomeningeal metastasis. Cancer Biother Radiopharm 2019;34:128-33.
73. Li YS, Jiang BY, Yang JJ, Zhang XC, Zhang Z, et al. Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung: a new medium of liquid biopsy. Ann Oncol 2018;29:945-52.
74. Ma C, Zhang J, Tang D, Ye X, Li J, et al. Tyrosine kinase inhibitors could be effective against non-small-cell lung cancer brain metastases harboring uncommon EGFR mutations. Front Oncol 2020;10:224.
75. Ma C, Yang X, Xing W, Yu H, Si T, et al. Detection of circulating tumor DNA from non-small cell lung cancer brain metastasis in cerebrospinal fluid samples. Thorac Cancer 2020;11:588-93.
76. Nevel KS, DiStefano N, Lin X, Skakodub A, Ogilvie SQ, et al. A retrospective, quantitative assessment of disease burden in patients with leptomeningeal metastases from non-small-cell lung cancer. Neuro Oncol 2020;22:675-83.
77. Oechsle K, Lange-Brock V, Kruell A, Bokemeyer C, de Wit M. Prognostic factors and treatment options in patients with leptomeningeal metastases of different primary tumors: a retrospective analysis. J Cancer Res Clin Oncol 2010;136:1729-35.
78. Wolf A, Donahue B, Silverman JS, Chachoua A, Lee J K, et al. Stereotactic radiosurgery for focal leptomeningeal disease in patients with brain metastases. J Neurooncol 2017;134:139-43.
79. Gani C, Müller AC, Eckert F, Bender B, Pantazis G, et al. Outcome after whole brain radiotherapy alone in intracranial leptomeningeal carcinomatosis from solid tumors. Strahlenther Onkol 2012;188:148-53.
80. Ozdemir Y, Yildirim BA, Topkan E. Whole brain radiotherapy in management of non-small-cell lung carcinoma associated leptomeningeal carcinomatosis: evaluation of prognostic factors. J Neurooncol 2016;129:329-35.
81. Brower JV, Saha S, Rosenberg SA, Hullett CR, Robins HI, et al. Management of leptomeningeal metastases: prognostic factors and associated outcomes. J Clin Neurosci 2016;27:130-7.
82. Hermann B, Hültenschmidt B, Sautter-Bihl ML. Radiotherapy of the neuroaxis for palliative treatment of leptomeningeal carcinomatosis. Strahlenther Onkol 2001;177:195-9.
83. Devecka M, Duma MN, Wilkens JJ, Kampfer S, Borm KJ, et al. Craniospinal irradiation(CSI) in patients with leptomeningeal metastases: risk-benefit-profile and development of a prognostic score for decision making in the palliative setting. BMC Cancer 2020;20:501.
84. Yang TJ, Wijetunga NA, Yamada J, Wolden S, Mehallow M, et al. Clinical trial of proton craniospinal irradiation for leptomeningeal metastases. Neuro Oncol 2020;noaa152.
85. Central Nervous System Cancers: leptomeningeal Metastases (2020). v.2.2020. Available from: http://www.nccn.org. [Last accessed on 21 Oct 2020].
86. Montes de Oca Delgado M, Cacho Díaz B, Santos Zambrano J, Guerrero Juárez V, López Martínez M S, et al. The comparative treatment of intraventricular chemotherapy by ommaya reservoir vs. lumbar puncture in patients with leptomeningeal carcinomatosis. Front Oncol 2018;8:509.
87. Zairi F, Le Rhun E, Bertrand N, Boulanger T, Taillibert S, et al. Complications related to the use of an intraventricular access device for the treatment of leptomeningeal metastases from solid tumor: a single centre experience in 112 patients,. J Neurooncol 2015;124:317-23.
88. Grossman SA, Finkelstein DM, Ruckdeschel JC, Trump DL, Moynihan T, et al. Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously untreated neoplastic meningitis. Eastern Cooperative Oncology Group. J Clin Oncol 1993;11:561-9.
89. Hitchins RN, Bell DR, Woods RL, Levi JA. A prospective randomized trial of single-agent versus combination chemotherapy in meningeal carcinomatosis. J Clin Oncol 1987;5:1655-62.
90. Glantz MJ, Jaeckle KA, Chamberlain MC, Phuphanich S, Recht L, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res 1999;5:3394-402.
91. Boogerd W, Hart AA, van der Sande JJ, Engelsman E. Meningeal carcinomatosis in breast cancer. Prognostic factors and influence of treatment. Cancer 1991;67:1685-95.
92. Lin Y, Li H, Huang M, Guo A, Yin Z. Use of ommaya reservoirs to deliver pemetrexed in leptomeningeal metastasis from non-small cell lung cancer: a case report and review of the literature. Zhongguo Fei Ai Za Zhi 2019;22:546-50.
93. Wu YL, Zhou L, Lu Y. Intrathecal chemotherapy as a treatment for leptomeningeal metastasis of non-small cell lung cancer: a pooled analysis. Oncol Lett 2016;12:1301-14.
94. Park JH, Kim YJ, Lee JO, Lee KW, Kim JH, et al. Clinical outcomes of leptomeningeal metastasis in patients with non-small cell lung cancer in the modern chemotherapy era. Lung Cancer 2012;76:387-92.
95. Ariyasu R, Horiike A, Koyama J, Saiki M, Sonoda T, et al. Efficacy of bevacizumab and erlotinib combination for leptomeningeal carcinomatosis after failure of erlotinib. Anticancer Drugs 2017;28:565-7.
96. Li Y S, Jiang BY, Yang JJ, Tu HY, Zhou Q, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol 2016;11:1962-9.
97. Kuiper JL, Hendriks LE, van der Wekken AJ, de Langen AJ, Bahce I, et al. Treatment and survival of patients with EGFR-mutated non-small-cell cancer and leptomeningeal metastasis: a retrospective cohort analysis. Lung Cancer 2015;89:255-61.
98. Lee E, Keam B, Kim DW, Kim TM, Lee SH, et al. Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small-cell lung cancer. J Thorac Oncol 2013;8:1069-74.
99. Yang H, Yang X, Zhang Y, Liu X, Deng Q, et al. Erlotinib in combination with pemetrexed/cisplatin for leptomeningeal metastases and cerebrospinal fluid drug concentrations in lung adenocarcinoma patients after gefitinib failure. Target Oncol 2015;10:135-40.
100. Kawamura T, Hata A, Takeshita J, Fujita S, Hayashi M, et al. High-dose erlotinib for refractory leptomeningeal metastases after failure of standard-dose EGFR-TKIs. Cancer Chemother Pharmacol 2015;75:1261-6.
101. Jackman DM, Cioffredi LA, Jacobs L, Sharmeen F, Morse LK, et al. A phase I trial of high dose gefitinib for patients with leptomeningeal metastases from non-small cell lung cancer. Oncotarget 2015;6:4527-36.
102. Liao BC, Lee JH, Lin CC, Chen YF, Chang CH, et al. Epidermal growth factor receptor tyrosine kinase inhibitors for non-small-cell lung cancer patients with leptomeningeal carcinomatosis. J Thorac Oncol 2015;10:1754-61.
103. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017;376:629-40.
104. Yang JCH, Kim SW, Kim DW, Lee JS, Cho BC, et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: the bloom study. J Clin Oncol 2020;38:538-47.
105. Saboundji K, Auliac JB, Perol M, François G, Janicot H, et al. Efficacy of osimertinib in EGFR mutated non-small-cell lung cancer with leptomeningeal metastases pretreated with EGFR tyrosine kinase inhibitors. Target Oncol 2018;13:501-7.
106. Ahn MJ, Chiu CH, Cheng Y, Han JY, Goldberg SB, et al. Osimertinib for patients with leptomeningeal metastases associated with EGFR T790M-positive advanced NSCLC: the AURA leptomeningeal metastases analysis. J Thorac Oncol 2020;15:637-48.
107. Park S, Lee MH, Seong M, Kim ST, Cho BC, et al. A phase II, multicenter, two cohort study of 160 mg osimertinib in EGFR T790M-positive non-small cell lung cancer patients with brain metastases or leptomeningeal disease who progressed on prior EGFR TKI therapy. Ann Oncol 2020;S0923-7534(20)39927-0.
108. Okuno T, Arakawa S, Yoshida T, Ohe Y. Efficacy of osimertinib in a patient with leptomeningeal metastasis and EGFR uncommon S768I mutation. Lung Cancer 2020;143:95-6.
109. Ahn MJ, Kim DW, Kim TM, Lin CC, Ratnayake J, et al. Phase I study of AZD3759, a CNS penetrable EGFR inhibitor, for the treatment of non-small-cell lung cancer (NSCLC) with brain metastasis (BM) and leptomeningeal metastasis (LM). J Clin Oncol 2016;9003.
110. Cho BC, Ahn M, Lee J, Kim DW, Kim SW, et al. Phase I study (BLOOM) of AZD3759, a BBB penetrable EGFR inhibitor, in EGFRm NSCLC patients with leptomeningeal metastasis (LM) who progressed after other anticancer therapy. J Clin Oncol 2017;2069. (abstr)
111. Macias A, Neninger E, Santiesteban E, Boland W, Nicacio L, et al. 505 POSTER preliminary results of a phase II clinical trial of the anti EGFR monoclonal antibody Nimotuzumab in combination with whole brain radiation therapy in patients diagnosed with advanced non-small cell lung cancer tumors unresectable brain metastases. Eur J Cancer Suppl 2008;6:160-1.
112. Xu H, Zhou L, Lu Y, Su X, Cheng P, et al. Dual targeting of the epidermal growth factor receptor using combination of nimotuzumab and erlotinib in advanced non-small-cell lung cancer with leptomeningeal metastases: a report of three cases. Onco Targets Ther 2020;13:647-56.
113. Costa DB, Shaw AT, Ou SH, Solomon BJ, Riely GJ, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol 2015;33:1881-8.
114. Ahn HK, Han B, Lee SJ, Lim T, Sun SM, et al. ALK inhibitor crizotinib combined with intrathecal methotrexate treatment for non-small cell lung cancer with leptomeningeal carcinomatosis. Lung Cancer 2012;76:253-4.
115. Crinò L, Ahn MJ, De Marinis F, Groen HJM, Wakelee H, et al. Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND-2. J Clin Oncol 2016;34:2866-73.
116. Shaw AT, Kim TM, Crinò L, Gridelli C, Kiura K, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017;18:874-86.
117. Arrondeau J, Ammari S, Besse B, Soria JC. LDK378 compassionate use for treating carcinomatous meningitis in an ALK translocated non-small-cell lung cancer. J Thorac Oncol 2014;9:e62-3.
118. Dudnik E, Siegal T, Zach L, Allen AM, Flex D, et al. Durable brain response with pulse-dose crizotinib and ceritinib in ALK-positive non-small cell lung cancer compared with brain radiotherapy. J Clin Neurosci 2016;26:46-9.
119. Chow LQ, Barlesi F, Bertino EM, Branle F, Shi M, et al. Results of the ASCEND-7 phase II study evaluating ALK inhibitor ceritinib in patients with ALK+ non-small cell lung cancer metastatic to the brain. ESMO Congress 2019. Abstract 1478O. Available from: https://www.annalsofoncology.org/article/S0923-7534(19)59686-7/fulltext. [Last accessed on 22 Oct 2020].
120. Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390:29-39.
121. Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 2017;377:829-38.
122. Gainor JF, Sherman CA, Willoughby K, Logan J, Kennedy E, et al. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J Thorac Oncol 2015;10:232-6.
123. Ou SH, Sommers KR, Azada MC, Garon EB. Alectinib induces a durable (>15 months) complete response in an ALK-positive non-small cell lung cancer patient who progressed on crizotinib with diffuse leptomeningeal carcinomatosis. Oncologist 2015;20:224-6.
124. Gainor JF, Chi AS, Logan J, Hu RL, Oh KS, et al. Alectinib dose escalation reinduces central nervous system responses in patients with anaplastic lymphoma kinase-positive non-small cell lung cancer relapsing on standard dose alectinib. J Thorac Oncol 2016;11:256-60.
125. Gettinger SN, Bazhenova LA, Langer CJ, Salgia R, Gold KA, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol 2016;17:1683-96.
126. Gaye E, Geier M, Bore P, Guilloïque M, Lucia F, et al. Intra-cranial efficacy of brigatinib in an ALK-positive non-small cell lung cancer patient presenting leptomeningeal carcinomatosis. Lung Cancer 2019;133:1-3.
127. Califano R, Hochmair MJ, Gridelli C, Delmonte A, Camidge DR. Brigatinib (BRG) vs crizotinib (CRZ) in the phase III ALTA-1L trial. Ann Oncol 2019;30:ii41.
128. Pellerino A, Buffoni L, Rudà R, Soffietti R. Complete response of spinal metastases from non-small cell lung cancer with ALK inhibitors. Neurology 2019;93:217-9.
129. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123-35.
130. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627-39.
131. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50.
132. Barlesi F, Keunchil P, Ciardiello F, von Pawel J, Gadgeel S, et al. Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in advanced NSCLC. ESMO 2016;LBA44-PR.
133. Hulsbergen AFC, Mammi M, Nagtegaal SHJ, Lak AM, Kavouridis V, et al. Programmed death receptor ligand one expression may independently predict survival in patients with non-small cell lung carcinoma brain metastases receiving immunotherapy. Int J Radiat Oncol Biol Phys 2020;S0360-3016(20)31036-1.
134. Gion MRJ, Caramella C, Soria J, Soria JC, Besse B, et al. Symptomatic leptomeningeal metastasis improvement with nivolumab in advanced non-small celll lung cancer patient. Lung Cancer 2017;108:72-4.
135. Dudnik E, Yust-Katz S, Nechushtan H, Goldstein DA, Zer A, et al. Intracranial response to nivolumab in NSCLC patients with untreated or progressing CNS metastases. Lung Cancer 2016;98:114-7.
136. Hendriks LEL, Bootsma G, Mourlanette J, Henon C, Mezquita L, et al. Survival of patients with non-small cell lung cancer having leptomeningeal metastases treated with immune checkpoint inhibitors. Eur J Cancer 2019;116:182-9.
137. Brastianos PK, Lee EQ, Cohen JV, Tolaney SM, Lin NU, et al. Publisher correction: single-arm, open-label phase 2 trial of pembrolizumab in patients with leptomeningeal carcinomatosis. Nat Med 2020; doi: 10.1038/s41591-020-0978-1.
138. Salvador E, Shityakov S, Förster C. Glucocorticoids and endothelial cell barrier function. Cell Tissue Res 2014;355:597-605.
139. Bebawy JF. Perioperative steroids for peritumoral intracranial edema: a review of mechanisms, efficacy, and side effects. J Neurosurg Anesthesiol 2012;24:173-7.
140. Suzuki T, Sugimoto M, Koyama H, Mashimo T, Uchida I, et al. Inhibitory effect of glucocorticoids on human-cloned 5-hydroxytryptamine3A receptor expressed in xenopus oocytes. Anesthesiology 2004;101:660-5.
141. Ho CM, Ho ST, Wang JJ, Tsai SK, Chai CY. Dexamethasone has a central antiemetic mechanism in decerebrated cats. Anesth Analg 2004;99:734-9.
142. Roth P, Happold C, Weller M. Corticosteroid use in neuro-oncology: an update. Neurooncol Pract 2015;2:6-12.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Pellerino A, Internò V, Muscolino E, Mo F, Bruno F, Pronello E, Franchino F, Soffietti R, Rudà R. Leptomeningeal metastases from non-small cell lung cancer: state of the art and recent advances. J Cancer Metastasis Treat 2020;6:41. http://dx.doi.org/10.20517/2394-4722.2020.80
AMA Style
Pellerino A, Internò V, Muscolino E, Mo F, Bruno F, Pronello E, Franchino F, Soffietti R, Rudà R. Leptomeningeal metastases from non-small cell lung cancer: state of the art and recent advances. Journal of Cancer Metastasis and Treatment. 2020; 6: 41. http://dx.doi.org/10.20517/2394-4722.2020.80
Chicago/Turabian Style
Pellerino, Alessia, Valeria Internò, Erminia Muscolino, Francesca Mo, Francesco Bruno, Edoardo Pronello, Federica Franchino, Riccardo Soffietti, Roberta Rudà. 2020. "Leptomeningeal metastases from non-small cell lung cancer: state of the art and recent advances" Journal of Cancer Metastasis and Treatment. 6: 41. http://dx.doi.org/10.20517/2394-4722.2020.80
ACS Style
Pellerino, A.; Internò V.; Muscolino E.; Mo F.; Bruno F.; Pronello E.; Franchino F.; Soffietti R.; Rudà R. Leptomeningeal metastases from non-small cell lung cancer: state of the art and recent advances. J. Cancer. Metastasis. Treat. 2020, 6, 41. http://dx.doi.org/10.20517/2394-4722.2020.80
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 42 clicks
Cite This Article 42 clicks


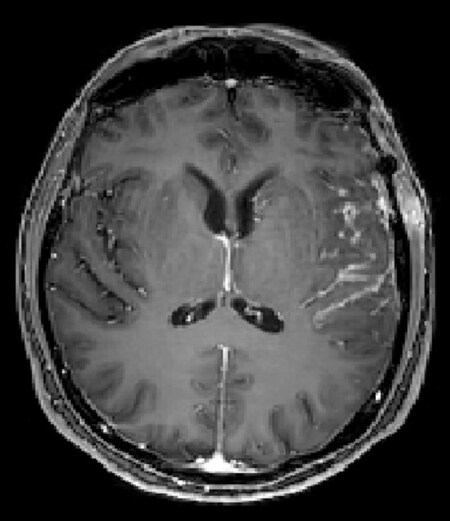
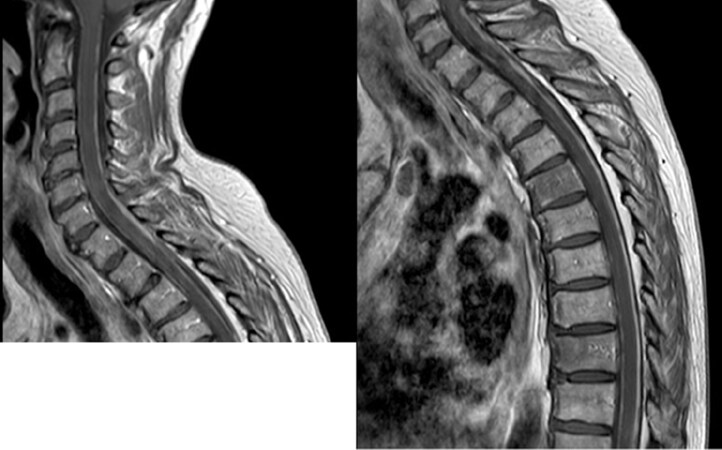
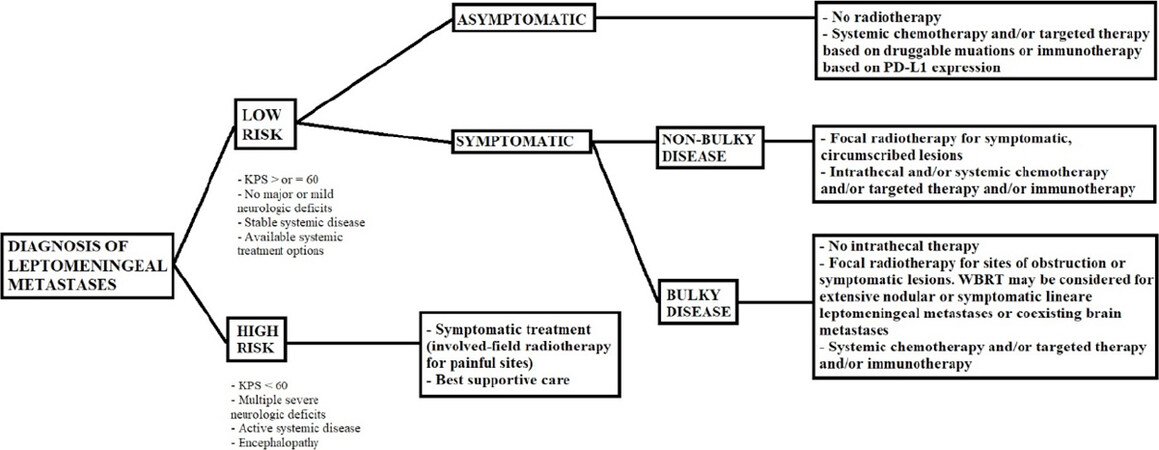









Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.