Isoflavone research towards healthcare applications
Abstract
A survey of the current literature on natural isoflavones and their biological activity is presented. This subcategory of a large group of plant polyphenolics has particular characteristics, structural as well as pharmacological, which makes it suitable for discussion of pleiotropic activities of phytochemicals and their exploitation in healthcare, beyond the concept of selectively targeted new drugs for narrow therapeutic indication.
Keywords
Introduction
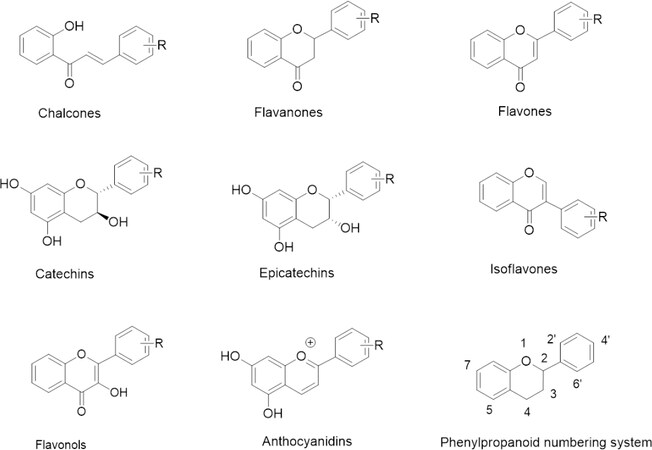
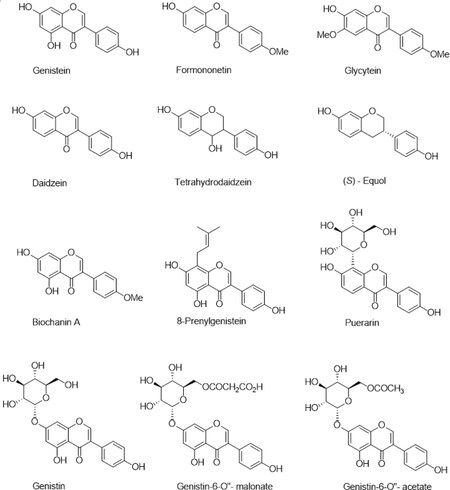
Aging populations inevitably suffer from chronic pathologic conditions recognized as metabolic syndrome, cancers, and neurodegenerations, with projections of ever-increasing burden on future healthcare budgets and service providers[1,2]. Modern medicine is constantly gaining efficient new tools based on advances in molecular biology and genetic engineering, but they are used more for cancer biology research, diagnostics and experimental procedures than in routine clinical therapeutic interventions. These typically rely on the administration of pharmacologically active preparations provided by the pharmaceutical industry, via a lengthy and exorbitantly costly multistage process of drug discovery and development. One of the most outstanding problems facing contemporary human healthcare, even in most affluent societies, is cancer treatment, since oncological morbidity and mortality continue to be leading causes of death, projecting a gloomy epidemiological forecast for the future[1,2]. Despite cancer pharmacology and the corresponding drug category started from synthetic alkylating agents, the overall pool of contemporary oncological therapeutics contains a very high proportion of natural products (NP) - native or slightly modified phytochemicals (notable examples include: anthracyclines, bis-indolyl alkaloids of Vinca and Catharanthus, diterpene taxanes, marine nucleoside analogs, podophyllotoxins, topotecans, etc.)[3-6]. Through pharmacognosy, every newly explored class of secondary metabolites was at first perceived as a collection of drug leads, based on the rich tradition of ethnopharmacology, which used to secure a natural remedy for every ailment. After a period of high hopes connected with high throughput chemical syntheses as source of new pharmaceuticals, NPs appear to offer better new drug leads, as well as better chances for successful chemopreventive interventions[7-10], provided support from cheminformatics and bioinformatics is properly applied. Presently, concerning very large but limited resources of NPs, we know what we do not know. With the number of species estimated at ca. 350 thousand, the number of secondary metabolites probably tops one million chemical entities; however, present lists of identified compounds from biological sources barely exceed 250 thousand in total[11-13], and knowledge of their biological activities is scant and fragmentary. The structural diversity of naturally derived chemicals is of particular value because of their intrinsic biocompatibility, indicating structure - biological activity relationships, essential for medicinal chemistry and novel drug design. We can imagine chemical space as a collection accommodating not only compounds from all databases but in fact all possible chemical structures (estimated at ca. 1060 in total), which can be navigated in search of structure clusters, featuring compounds of desired biological activities and acceptable physicochemical properties. Currently available cheminformatics tools make such searches possible, and new artificial intelligence (AI) and machine learning (ML) methodologies help to turn any collection of chemical structures (such as a class of phytochemicals) into a big data resource, through an extensive parametrization of its elements. Such an operation allows the substitution of some expensive biological activity testing with the property assessment by in silico modeling. Thus, a large pool of phytochemical metabolites can be conveniently segmented into subcategories of compounds that are privileged by featuring some desirable parameters, such as affinity to selected molecular targets[14,15]. It is generally believed that plant polyphenolics constitute a collection of metabolites with relatively high chemical affinity to peptides and proteins, representing a rich pool of prospective drug leads. The group is large and very heterogeneous, biogenetically and structurally. Although it has provided many contemporary drugs via traditional pharmacology efforts comprising target-based and structure-based searches[4-6], we prefer to select a smaller and more structurally consistent NP group for our assessment of prospective phytochemicals valued for therapy and prevention. Flavonoids can be chosen as a representative group of plant phenolics, having medium size (approx. 10 thousand), well-defined biogenesis, and structural similarity, which nevertheless contains considerable amount of native diversity and ample room for its expansion, via chemical or biotechnological derivatization[16-18]. Flavonoid subcategories [Figure 1] feature very interesting pharmacological activities, exemplified in a small and structurally distinct group of isoflavones [Figure 2], important for human nutrition as constituents of the leading agricultural crop - soybeans[19-21]. In our opinion, focus on isoflavone research serves well to illustrate changing trends in the role of phytochemicals in the interface between official academic medicine, less regulated segments of healthcare, and professional nutrition sciences. However, for a closer look and better perception of isoflavones, their placement within a wider context of the biogenetic family of flavonoids seems advisable.
Flavonoid pharmacology outlook
The name flavones (from Latin word for yellow; later expanded to flavonoids to accommodate more structural variety) was coined by S. Kostanecki for a group of yellow plant dyes containing a chromane nucleus with aromatic (phenolic) substituents, towards the end of the XIXth century[22,23]. Early stages of their biological activity studies were expertly summarized in numerous monographic works[24-26]. Roles of flavonoids in plant physiology and ecology are now better understood and can be related to the biology of other organisms, including humans[27-30]. Historically, interest in flavonoids was limited to a narrow field of natural pigments and their occurrence and chemistry. In the 1930s, amid the race for vitamin C resources, Albert Szent-Györgyi noticed that citrus and green pepper flavonoids (then called “citrin” and proposed to be included in a vitamin category) greatly enhanced the antiscorbutic activity of L-ascorbic acid[31], which commenced lasting interest in their pharmacological activity. Flavonoids constitute a class of ubiquitous plant metabolites, which stem from common biogenetic transformation, combining shikimic acid - phenylalanine, and acetyl CoA pathways, resulting in the assembly of a phenylpropanoid scaffold ending up as 2- or 3-phenylchromane derivatives, categorized as: flavones, flavanones, catechins, anthocyanidins, and isoflavones[26,27]. These structures tend to be further diversified biocatalytically by oxidation, hydroxyl group alkylation (methylation, isoprenylation), and/or glycosylation, but also by C-C coupling reactions, which lead to oligomerization and polymerization, producing in turn a wide array of structural variety, and consequently a very complex matrix of biological activity (Figure 1 shows low-molecular-weight flavonoids only). Presently, it is clear that the biogenesis of flavonoids, which is common throughout the plant kingdom, can be considered one of the most significant evolutionary developments, occurring first as a protective environmental adaptation and further adopted for many other functions[32].
In the last decades, hundreds of new papers devoted to the biological activity of plant flavonoids (including review papers) have appeared in the scientific literature. The majority have dealt with preclinical pharmacology, studied using molecular and subcellular models, as well as cell lines and experimental animals[33-40]. Antioxidant activity and anti-inflammatory pathways were among the favorite study subjects[28]. Cancer prevention and anticancer activity evoked continuous interest[20,30] and the proportion of papers heralding the intimate connection between flavonoids and cancer is remarkably high[37-41]. Comments on flavonoid systemic activity point out their limited bioavailability[42,43], which offers poor prognosis for clinical efficacy. Despite encouraging results in many preclinical studies, no flavonoid drug candidate could be seen advancing beyond early stages of clinical trials until very recently. A breakthrough came from the side of dihydrochalcone - phloretin glycoside, (phlorizin, known since the mid-XIXth century), which surfaced as an antidiabetic drug candidate, featuring effective inhibition of GLUT family D-glucose transporters. Phlorizin turned out to be metabolically unstable, because susceptibility to hydrolases; this ultimately led to the synthesis of C-glycosyl analogs, which turned into a new generation of antidiabetics, started by the launch of dapagliflozin in 2012[44-46].
Isoflavone estrogens and food connection
One immediate reflection on the massive amount of research accumulated in recent decades on flavonoid biological activity, is that of the large discrepancy in adopted quality standards, which even became a subject of separate study[47,48]. Strict adherence to accuracy in measurement and good laboratory practices should certainly help in interdisciplinary discussions of somewhat fuzzy notions such as research material specification, molecular similarity, antioxidant properties, estrogenicity, chemoprevention, etc. Despite the constant refinement of scientific information through the exchange of peer opinion, some statements that lack factual support can persist in literature for decades. Nearly all papers tackling the subject of isoflavone activity underline genistein similarity to 17-β-estradiol (E2), offering only a single parameter - distance of ca. 12Å between two hydroxyl groups in both ER ligands for support of that opinion. Such superficial statements disregard the fact that molecular similarity is an important issue in current cheminformatics as well as medicinal chemistry, which calls for quantification in reference to a selected model[49-51]. It should not be overlooked that molecular parametrization systems, which are compatible with AI, ML, and neural networks, must take over to be able to cope with big data. Coping with expanding databases, which store astronomical quantities of information on the structures of chemical and biological objects and their parameters and interrelations, quickly become essential for in silico drug design and initial evaluation[52-54].
Natural isoflavones belong to the phenylpropanoid (flavonoid) category through a common biogenetic pathway and share a considerable part of chemical and biochemical characteristics with the large group of plant polyphenols, also these being from other branches of aromatic secondary metabolite origin. Their fundamental structural difference from other subcategories of flavonoids (which are 2-aryl chromones) resides in the phenylpropanoid ABC ring system connectivity [Figure 2], which stems from isoflavone synthase action, transferring the aromatic B ring to the C-3 atom of the AC chromenone system. Unlike ubiquitous flavones, flavonols and anthocyanidins which are widespread throughout the plant kingdom, isoflavones occur mainly in the family Fabaceae, which is particularly important for animal feed and human nutrition[55].
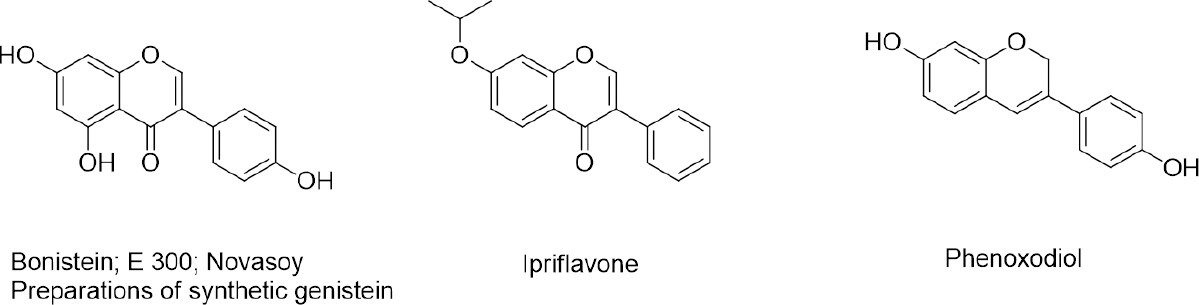
An interest in biological activity of isoflavones initiated when seasonal intake of Trifolium subterraneum, containing an isoflavone fraction rich in formononetin, was identified as the cause of sheep fertility problems (called “clover disease”) in Western Australia around 1940[56,57]. After thorough veterinarian investigation which followed, isoflavones were classified as phytoestrogens and occasionally even included in a category of endocrine disruptors (negative classification of environmental industrial pollutants with phenolic characteristics). Later, this seemingly local problem, seriously affected the perception of soy as principal agricultural crop and important source of animal feed and human food[58,59]. Soybeans contain on average more than one mg/g of isoflavones (genistein, daidzein, and glycitein and their glycosides; Figure 2) which during regular oil separation-oriented industrial process end up in the protein fraction and further in soy flour-derived products[60,61]. Phytoestrogenic food components may be considered beneficial for some consumer segments (such as women in their post-menopausal period of life) but may cause serious concerns for others (infants fed with soy-based formula, prepubertal youth, cancer patients, etc.)[62-64]. This warranted basic pharmacological research which started soon after the discovery of estrogen receptors (ERs), and its results are a matter of continuous reassessment, critical review and constant debate. Isoflavones such as the soy constituent genistein are proven ER ligand subtypes, although with considerably lower affinity than the natural substrate: 17-β-estradiol. Because they can attain much higher concentrations than natural estrogens, competition for the ER binding site is possible, as proven by radioisotope-labeling experiments. However, the ligand-receptor affinity issue is only a minute part of the estrogenic effect complexity. Bioavailability, pharmacokinetics and metabolism (including microbiome biotransformation) can make a dramatic difference on a systemic level. Pharmacodynamically, phytoestrogens can exert their effects via different mechanisms and pathways. Ligands that can directly enter cells and internalize into the nucleus bind to ER and initiate ERE (estrogen response elements) responses effects via interactions with DNA (genomic mechanism). Alternatively, non-genomic signaling takes place when ligands bind to membrane receptors and start intracellular kinase cascades. Genistein (4’, 5, 7-trihydroxyisoflavone), one of the most studied NPs, is a good example of a partially agonistic ligand of both ERs, which are differentially expressed in various tissues and can regulate antagonistically such basic processes as cell cycle, proliferation or apoptosis, which makes it very difficult to predict its chemopreventive or therapeutic intervention effect[65]. Additionally, adequate analytical techniques such as HPLC/MS/MS for proper quantification of phytoestrogen in a plant/food matrix, assessment of its pharmacokinetic parameters from serum analysis and also systemic metabolite determination, have become available relatively recently. When the major FDA decision concerning soy nutrient was undertaken, recommending everyday 25 g soy protein administration for cholesterol level control, neither proteins nor isoflavones which it contained, were specified[66]. Currently, when any plant-derived material can be analyzed with great accuracy, soy protein isolates and concentrates, with varying isoflavone content declared, remain in the segment of nutraceuticals with countless estrogenic preparations based on herbal extracts. Soy is grown in huge amounts of 300 million tons harvested annually, which contains at least 1 g of isoflavone fraction per kilogram of crop. Numerous technical processes allowing for isoflavone isolation exist, practically ready for implementation[25-27]. Nevertheless, medicinal research materials, which are on a drug candidate evaluation path towards clinical trials (aiming at validation of isoflavones as preventive or therapeutic agents in bone health, oncological problems or neurodegeneration) are recently more often synthetic than of natural origin. Thus, synthetic genistein (BonisteinTM) was registered for assistance of bone health and to stop bone loss in postmenopausal women[67]. As a phytoestrogen, genistein have been validated for a number of targets other than ER, such as NF-κB, EGFR, CDK, KIF20A, PLK1, and AR, in molecular and cellular models[68]. Even at that preclinical level, there are inconsistencies in results of cell cycle arrest, kinase inhibition data and proliferation of cells with various ER status[65]. Genistein has been the subject of many randomized clinical trials in connection with postmenopausal health, various cancers[69] and neurodegenerating San Filippo disease, which is genetically conditioned faulty storage of glycosaminoglycans[70][Figure 3].
New hurdles appear when it comes to clinical trials, plagued by problems of low isoflavone solubility and bioavailability, and high genetic as well as metabolic variety among clinical trial participants. Generally, there seems to be a problem of design and crisis of credibility in clinical trials, which affects many drug candidates from the phytochemical pool, especially in the anticancer field[64,65,68]. Soy products for human food use are generally considered beneficial for health, although some controversies concerning its estrogenic effects persist. Technically, soy isoflavone-free products could be obtainable by relatively minor soybean process modifications but GRAS status and good market standing of soy nutraceuticals provide arguments against such decision. Reflection on advancement of evidence-based medicine requirements seem to indicate that the chances of further progress of soy isoflavone phytoestrogens towards registered medicine status are very slim; on the other hand, their existence among nutraceuticals and on the complementary and alternative medicine market is unquestionable. Also, rational nutrition is a large forum for innovation aiming at improvement of healthcare by prevention rather than therapeutic intervention.
Nutrition habits are already considerably influenced by the availability of high-quality scientific knowledge obtainable through the internet and open sources. Future food choices undoubtedly are going to reflect availability of both scientifically validated food and health data and relevant commercially available materials for personalized diet.
New developments in genistein research
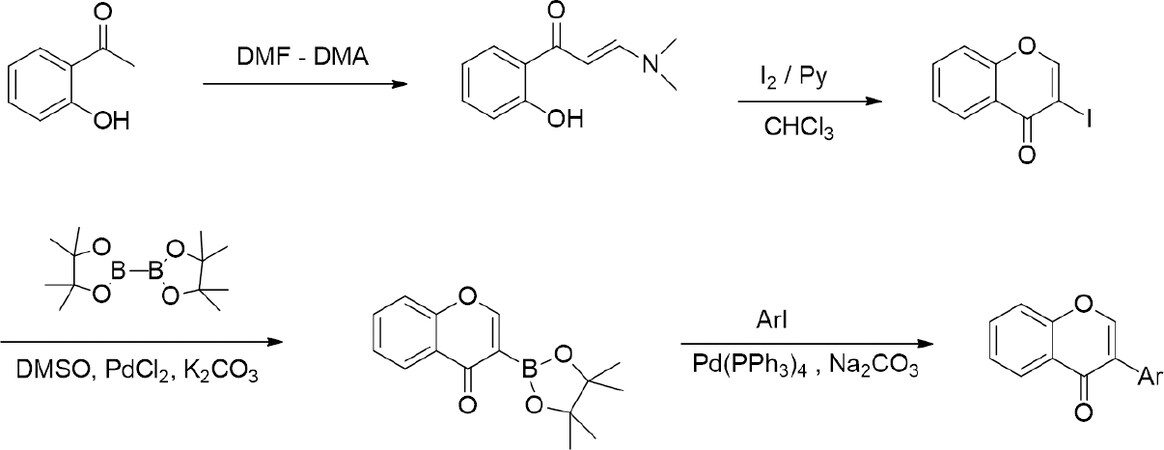
An example of turning a XIXth century phytochemical discovery (apple tree glycoside - phlorizin) into XXIst century medicinal and pharmaceutical hit: a new generation of antidiabetic drugs - gliflozins[44-46] cited above, indicates clearly that the potential of combined chemical and biological sciences for significant industrial applications is far from being exhausted. Consequently, research on genistein, which is a good drug lead but poor drug candidate (low solubility and bioavailability, far from optimal metabolism) is going strong[71], not only in academic environment. The main genistein shortcoming - low bioavailability, is being addressed in two ways: by chemical derivatization, and by the preparation of a suitable availability-enhancing formulation, preferably nanotechnological. Two ways of genistein structure modification are being energetically pursued: one uses phenolic groups for pharmacophore extension via alkylation followed by further functionalization[72]; the second aims at glycoconjugation through chemical glycosylation, which offers ample room for modulating molecular polarity balance[17,21]. Both directions in principle exploit natural isoflavone structure as a starting point, but new synthetic development based on de novo assembly of the isoflavone skeleton significantly expands the scope of structural modifications [Scheme 1].
Newly synthesized genistein derivatives revealed improved bioavailability and very promising pharmacodynamic profiles, but their investigation stopped at early preclinical evaluation. Meanwhile, a project that investigated the radiation-protecting activity of genistein, which obtained state support for research on nanoformulation in the USA, achieved radical improvement in isoflavone bioavailability by preparing nanosuspensions of its particles of ca. 200 nm. The synthetic genistein preparation BIO 300 is presently the subject of two investigational new drug (IND) applications at the FDA[73-75].
Conclusion
Isoflavones, which constitute a well-defined subcategory of medicinally important large collection of plant phenolics, remain of interest as subjects of medicinal chemistry, biologically active food components, and nutraceuticals of increasing interest for functional food, complementary medicine and self-medication. Application of novel research tools (e.g., in bioinformatics, synthesis, nanotechnology) open new opportunities in healthcare R&D. Recent advancement of genistein to IND level as a protective agent against acute radiation syndrome heralded a breakthrough in overcoming the low bioavailability of active pharmaceutical ingredients, by the application of a suitable nanosuspension. This new pre-formulation solution is likely to revive some older clinical trial projects.
Declarations
Authors’ contributionsThe author contributed solely to the article.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestThe author declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2020.
REFERENCES
2. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941-53.
3. Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochimica et Biophysica Acta (BBA) - General Subjects 2013;1830:3670-95.
4. Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 2015;79:629-61.
5. Kaur V, Kumar M, Kumar A, Kaur K, Dhillon VS, Kaur S. Pharmacotherapeutic potential of phytochemicals: implications in cancer chemoprevention and future perspectives. Biomed Pharmacother 2018;97:564-86.
6. Sharifi-Rad J, Ozleyen A, Boyunegmez Tumer T, et al. Natural products and synthetic analogs as a source of antitumor drugs. Biomolecules 2019;9:679.
7. Dias DA, Urban S, Roessner U. A historical overview of natural products in drug discovery. Metabolites 2012;2:303-36.
8. Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov 2015;14:111-29.
9. Ertl P, Schuhmann T. Cheminformatics analysis of natural product scaffolds: comparison of scaffolds produced by animals, plants, fungi and bacteria. Mol Inform 2020;39:2000017.
10. Chen Y, Kirchmair J. Cheminformatics in natural product-based drug discovery. Mol Inform 2020:2000171.
11. Chen Y, Garcia de Lomana M, Friedrich NO, Kirchmair J. Characterization of the chemical space of known and readily obtainable natural products. J Chem Inf Model 2018;58:1518-32.
12. Ntie-kang F, Nyongbela KD, Ayimele GA, Shekfeh S. “Drug-likeness” properties of natural compounds. Phys Sci Rev 2019;4.
13. Saldívar-gonzález FI, Pilón-jiménez BA, Medina-franco JL. Chemical space of naturally occurring compounds. Phys Sci Rev 2019;4.
14. Luo Y, Cobb RE, Zhao H. Recent advances in natural product discovery. Curr Opin Biotechnol 2014;30:230-7.
15. Lagunin AA, Goel RK, Gawande DY, et al. Chemo- and bioinformatics resources for in silico drug discovery from medicinal plants beyond their traditional use: a critical review. Nat Prod Rep 2014;31:1585-611.
16. Biasutto L, Mattarei A, Sassi N, et al. Improving the efficacy of plant polyphenols. Anticancer Agents Med Chem 2014;14:1332-42.
17. Grynkiewicz G, Szeja W, Krzeczyński P, Rusin A. Hexenoses in design of glycoconjugates - from chemistry to function biological activity of genistein derivatives. Chem Biol Interface 2014;4:301-20.
18. Blakemore DC, Castro L, Churcher I, et al. Organic synthesis provides opportunities to transform drug discovery. Nature Chem 2018;10:383-94.
19. Messina MA. Brief historical overview of the past two decades of soy and isoflavone research. J Nutr 2010;140:1350S-4.
20. Ko KP. Isoflavones: chemistry, analysis, functions and effects on health and cancer. Asian Pac J Cancer Prev 2014;15:7001-10.
21. Szeja W, Grynkiewicz G, Rusin A. Isoflavones, their glycosides and glycoconjugates. Synthesis and biological activity. Curr Org Chem 2017;21:218-35.
22. Venkataraman K. Flavones and isoflavones. Progr Chem Org Nat Prod 1959;17:2-69.
23. Wong E. Structural and biogenetic relationships of isoflavonoids. Progr Chem Org Nat Prod 1970;28:1-73.
24. In: Catherine A, Rice-Evans C, Lester Packer L, editors. Flavonoids in Health and Disease Second Edition. Boca Raton, FL: CRC Press; 2003.
25. In: Grotewold E, editor. The Science of Flavonoids. New York: Springer Science + Business Media; 2006.
26. In: Andersen OM, Markham KR, editors. Flavonoids Chemistry, Biochemistry and Applications. Boca Raton, FL: CRC Taylor & Francis; 2006.
27. In: Keller RB, editor. Flavonoids: Biosynthesis, Biological Effects and Dietary Sources. New York: Nova Science Publishers, Inc.; 2009.
28. Vicente O, Bosatu M. Flavonoids: antioxidant compounds for plant defence...and for a healthy human diet. Not Bot Horti Agrobo 2018;46:14-21.
30. Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. Sci World J 2013;2013:1-16.
31. Perez-vizcaino F, Fraga CG. Research trends in flavonoids and health. Arch Biochem Biophys 2018;646:107-12.
32. Naoumkina MA, Zhao Q, Gallego-giraldo L, Dai X, Zhao PX, Dixon RA. Genome-wide analysis of phenylpropanoid defence pathways: phenylpropanoid defence pathways. Mol Plant Pathol 2010;11:829-46.
33. Prasad S, Phromnoi K, Yadav V, Chaturvedi M, Aggarwal B. Targeting inflammatory pathways by flavonoids for prevention and treatment of cancer. Planta Med 2010;76:1044-63.
34. Procházková D, Boušová I, Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011;82:513-23.
35. Romano B, Pagano E, Montanaro V, Fortunato AL, Milic N, Borrelli F. Novel insights into the pharmacology of flavonoids: pharmacology of flavonoids. Phytother Res 2013;27:1588-96.
36. Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines (Basel) 2018;5:93.
37. Ravishankar D, Rajora AK, Greco F, Osborn HMI. Flavonoids as prospective compounds for anti-cancer terapy. Int J Biochem Cell Biol 2013;45:2821-31.
38. Raffa D, Maggio B, Raimondi MV, Plescia F, Daidone G. Recent discoveries of anticancer flavonoids. Eur J Med Chem 2017;142:213-28.
39. Rodríguez-garcía C, Sánchez-quesada C, Gaforio JJ. Dietary flavonoids as cancer chemopreventive agents: an updated review of human studies. Antioxidants 2019;8:137.
40. Abotaleb M, Samuel S, Varghese E, et al. Flavonoids in cancer and apoptosis. Cancers 2019;11:28.
41. Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as anticancer agents. Nutrients 2020;12:457.
42. Thilakarathna S, Rupasinghe H. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013;5:3367-87.
43. Rodriguez-mateos A, Vauzour D, Krueger CG, et al. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Arch Toxicol 2014;88:1803-53.
44. Nicolle E, Souard F, Faure P, Boumendjel A. Flavonoids as promising lead compounds in type 2 diabetes mellitus: molecules of interest and structure-activity relationship. Curr Med Chem 2011;18:2661-72.
45. Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Asp Med 2013;34:121-38.
46. Aguillón AR, Mascarello A, Segretti ND, et al. Synthetic strategies toward SGLT2 Inhibitors. Org Process Res Dev 2018;22:467-88.
47. Balentine DA, Dwyer JT, Erdman JW, et al. Recommendations on reporting requirements for flavonoids in research. Am J Clin Nutr 2015;101:1113-25.
48. Harnly J. Importance of accurate measurements in nutrition research: dietary flavonoids as a case study. Adv Nutr 2016;7:375-82.
49. Maggiora G, Vogt M, Stumpfe D, Bajorath J. Molecular similarity in medicinal chemistry miniperspective. J Med Chem 2014;57:3186-204.
50. Popelier P, Smith P. QSAR models based on quantum topological molecular similarity. Eur J Med Chem 2006;41:862-73.
51. Renner S, Schneider G. Scaffold-hopping potential of ligand-based similarity concepts. ChemMedChem 2006;1:181-5.
52. Scalbert A, Andres-lacueva C, Arita M, et al. Databases on food phytochemicals and their health-promoting effects. J Agric Food Chem 2011;59:4331-48.
53. Wishart DS, Mandal R, Stanislaus A, Ramirez-Gaona M. Cancer metabolomics and the human metabolome database. Metabolites 2016;6:10.
54. Sebastian RS, Enns CW, Goldman JD, et al. New, publically available flavonoid data products: valuable resources for emerging science. J Food Compos Anal 2017;64:68-72.
56. Adams NR. Permanent infertility in ewes exposed to plant oestrogens. Aust Vet J 1990;67:197-201.
57. Adams NR. Detection of the effects of phytoestrogens on sheep and cattle. J Anim Sci 1995;73:1509-15.
58. Jefferson WN, Patisaul HB, Williams CJ. Reproductive consequences of developmental phytoestrogen exposure. Reproduction 2012;143:247-60.
59. Douglas C, Johnson S, Arjmandi B. Soy and its isoflavones: the truth behind the science in breast cancer. Anticancer Agents Med Chem 2013;13:1178-87.
60. Zaheer K, Humayoun Akhtar M. An updated review of dietary isoflavones: nutrition, processing, bioavailability and impacts on human health. Crit Rev Food Sci Nutr 2016;57:1280-93.
62. Ososki AL, Kennelly EJ. Phytoestrogens: a review of the present state of research. Phytother Res 2003;17:845-69.
63. Hooper L, Ryder J, Kurzer M, et al. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update 2009;15:423-40.
64. Douglas C, Johnson S, Arjmandi B. Soy and its isoflavones: the truth behind the science in breast cancer. Anticancer Agents Med Chem 2013;13:1178-87.
65. Chae HS, Xu R, Won JY, Chin YW, Yim H. Molecular targets of genistein and its related flavonoids to exert anticancer effects. Int J Mol Sci 2019;20:2420.
66. Sacks FM, Lichtenstein A, Van Horn L, et al. Soy protein, isoflavones, and cardiovascular health. Circulation 2006;113:1034-44.
67. Ullmann U, Bendik I, Flühmann B. Bonistein (synthetic genistein) a food component in development for a bone health nutraceutical. J Physiol Pharmacol 2005;56:79-95.
70. Banecka-Majkutewicz Z, Jakóbkiewicz-Banecka J, Gabig-Cimińska M, Węgrzyn A, Węgrzyn G. Putative biological mechanism of efficiency of substrate reduction therapies for mucopolisaccharidoses. Arch Immunol Ther Exp 2012;60:461-8.
71. Ferrari SM, Antonelli A, Guidi P, et al. Genotoxicity evaluation of the soybean isoflavone genistein in human papillary thyroid cancer cells. Study of its potential use in thyroid cancer therapy. Nutr Cancer 2019;71:1335-44.
72. Rusin A, Krawczyk Z, Grynkiewicz G, et al. Synthetic derivatives of genistein, their properties and possible applications. Acta Biochim Polon 2010;57:23-34.
73. Landauer MR, Harvey AJ, Kaytor MD, Day RM. Mechanism and therapeutic window of genistein nanosuspension to protect against hematopoietic-acute radiation syndrome. J Rad Res 2019;2019:308-17.
74. Singh VK, Seed TM. Bio 300: a promising radiation countermeasure under advanced development for acute radiation syndrome and the delayed effects of acute radiation exposure. Exp Opin Investig Drugs 2020;29:429-41.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Grynkiewicz G. Isoflavone research towards healthcare applications. J Cancer Metastasis Treat 2020;6:48. http://dx.doi.org/10.20517/2394-4722.2020.112
AMA Style
Grynkiewicz G. Isoflavone research towards healthcare applications. Journal of Cancer Metastasis and Treatment. 2020; 6: 48. http://dx.doi.org/10.20517/2394-4722.2020.112
Chicago/Turabian Style
Grynkiewicz, Grzegorz. 2020. "Isoflavone research towards healthcare applications" Journal of Cancer Metastasis and Treatment. 6: 48. http://dx.doi.org/10.20517/2394-4722.2020.112
ACS Style
Grynkiewicz, G. Isoflavone research towards healthcare applications. J. Cancer. Metastasis. Treat. 2020, 6, 48. http://dx.doi.org/10.20517/2394-4722.2020.112
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 10 clicks
Cite This Article 10 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.