Role of 18F-FDG PET/CT in detection of hematogenous metastases of advanced differentiated thyroid carcinoma: a systematic review and meta-analysis
Abstract
The aim of this study was to collect the evidence on the performance of 2-deoxy-2-[18F]fluoro-D-glucose (18F-FDG) Positron Emission Tomography/Computed Tomography (PET/CT) in terms of detection rate (DR) and diagnostic accuracy in identifying hematogenous metastases in patients with differentiated thyroid cancer and compare its performance with that of other imaging techniques. A comprehensive PubMed/MEDLINE database research was carried out to retrieve studies documenting the performance of 18F-FDG PET/CT in depicting hematogenous metastases in patients with differentiated thyroid cancer. Statistical analysis was performed on a per-patient and per-lesion basis. The literature search yielded 15 articles to be included in the systematic review. 18F-FDG PET/CT showed a pooled DR of 85.08% on a per-patient analysis and 89.70% on a per-lesion analysis. For bone lesions, a high DR (81.78%) was found for 18F-FDG PET/CT. For the sub-group analysis of lung lesions, pooled DR was 92.77% for 18F-FDG PET/CT, 95.02% for CT, and 64.93% for magnetic resonance imaging (MRI). On a per-patient analysis, 18F-FDG PET/CT demonstrated a pooled sensitivity (SS) of 87.3% [95% confidence interval (CI): 77.3%-94%] and a pooled specificity (SP) of 95.6% (95%CI: 87.6-99.1). On a per-lesion analysis (328 metastases), PET/CT showed a pooled SS and SP of 86.3% (95%CI: 73.5%-93.5%) and 93.4% (95%CI: 71.7%-98.8%) in the detection of hematogenous metastases. The presented systematic review and meta-analysis favor the use of 18F-FDG PET/CT in the detection of hematogenous metastases in patients with differentiated thyroid cancer. The limited literature warrants further studies to confirm our findings.
Keywords
Introduction
Primitive thyroid cancers can be divided into 2 main pathological entities: tumors originating from follicular cells and medullary cancer (arising from para-follicular C cells). The first group of cancers include differentiated (follicular and papillary carcinoma and their variants), poorly differentiated, and undifferentiated tumors (anaplastic carcinoma)[1].
After surgical removal of the tumor, due to the risk of disease recurrence, differentiated thyroid cancer (DTC) is followed-up by assessing serum thyroglobulin (Tg), whereas calcitonin and carcinoembryonic antigen are biochemical markers for postoperative surveillance in medullary thyroid cancer[2,3]. Overall, lymph node is the most frequent metastatic site for thyroid cancer. Nevertheless, patients with thyroid cancer may present also distant metastases in other organs due to hematogenous dissemination, especially to bone and lung. Patients with extranodal metastases usually present a worse prognosis compared to patients with metastases limited to lymph nodes[4,5]. It follows that it is extremely important to identify hematogenous metastases in these patients to better address clinical management.
Patients with history of DTC, who no longer respond to radioactive iodine (RAI) therapy, have developed RAI-Refractory DTC. The majority of these cases with advanced disease have a worse prognosis[6]. The use of 2-deoxy-2-[18F]fluoro-D-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) has proved beneficial in assessing recurrence of thyroid cancer in the case of a rise of Tg but no evidence of disease at 131Iodium (131I) whole-body scan[7,8]. This clinical scenario has been related to the so-called flip-flop phenomenon and appears to be associated with worse prognosis; thyroid cells, indeed, demonstrate an upregulation of glucose transporters (GLUT1) and a reduction of sodium-iodide symporters (NIS) when a dedifferentiation process occurs (from DTC towards a more aggressive histotype)[9].
In the literature, there are systematic reviews and meta-analyses documenting the accuracy of 18F-FDG PET/CT or 18F-FDG PET in assessing in patients with thyroid cancer with suspected recurrence, although these studies did not differentiate between lymph node and hematogenous metastases[10-12].
Due to the widespread availability of hybrid PET/CT scanners in current nuclear medicine facilities worldwide, we excluded from our systematic review studies involving the use of stand-alone PET scanners. Lee and coworkers recently demonstrated in a network meta-analysis the superiority of L-6-[18F]fluoro-3,4-dihydroxyphenylalnine) (18F-DOPA) over 18F-FDG in medullary thyroid cancer. We, therefore, decided to focus on thyroid cancer histotypes other than medullary[13].
The primary aim of this systematic review and meta-analysis was to collect the evidence on the detection rate (DR) and accuracy (AC) of 18F-FDG PET/CT in identifying hematogenous metastatic lesions in patients with advanced differentiated thyroid cancer on a per-patient and per-lesion basis. The secondary goal was the comparison of the diagnostic performance of 18F-FDG PET/CT with other imaging techniques.
Methods
The systematic review was conducted in accordance with the PRISMA guideline (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)[14].
Literature search
A comprehensive PubMed/MEDLINE database research was carried out by a researcher (N.Q.) to retrieve prospective or retrospective studies, aiming the identification of distant metastases in patients with thyroid cancer by means of 18F-FDG PET/CT. The following search string was used for the literature search: (“positron emission tomography” OR “PET” OR “positron emission tomography/computer tomography” OR “PET/CT”) AND [(“differentiated” OR “papillary” OR “follicular” OR “Hurthle cell” OR “anaplastic” OR “dedifferentiated” OR “poorly differentiated”) AND (“cancer” OR “carcinoma”)] AND (“fluorodeoxyglucose” OR “FDG”) AND “thyroid”.
The literature search was updated until 22 August 2020. No date limit or language restriction was applied. All identified references were exported to a reference management software (Endnote v. X7.5, Clarivate Analytics).
Study selection
An investigator screened the titles and abstracts of the retrieved entries. Only original articles were selected. After excluding duplicates and non-original articles, the full text of the remaining articles was retrieved to verify the following inclusion criteria: (1) a study cohort or a subset of a minimum 10 patients with differentiated thyroid cancer undergoing 18F-FDG PECT/CT; (2) presence of data regarding at least the detection rate of non-lymph node metastases; and (3) absence of other malignancies in the patient history. The choice of excluding articles with fewer than 10 patients and organs was made to mitigate the so-called small-study effect, for which, in brief, the observations could be due to methodological flaws, outcome reporting bias, and clinical heterogeneity.
The references of the retrieved articles were also screened for additional studies.
Data extraction
For our primary outcome (accuracy of 18F-FDG PET/CT), we selected articles from which it was possible to retrieve the number of true positive (TP), false negative (FN), false positive (FP), and true negative (TN) cases on a per-patient or per-lesion basis. Lesions were grouped according to the involved organ (e.g, lung or bone). Articles providing only the number of TP or FN cases were used only for the determination of the DR of hematogenous metastases on a per-patient and per-lesion basis.
For the secondary outcome (comparison of diagnostic performance of 18F-FDG PET/CT with other imaging tests), pooled DR of 18F-FDG PET/CT was compared with that of other imaging tests using N - 1 Chi-squared test[15].
Methodological quality assessment
The methodological quality of the studies was assessed using version 2 of the “Quality Assessment of Diagnostic Accuracy Studies” tool (QUADAS-2)[16], which comprises four domains: patient selection, index test, reference standard, and flow and timing. The concerns about the risk of bias or applicability were described as low, high, or unclear.
Statistical analysis
Statistical analysis was carried out using MedCalc Statistical Software version 19.1.3 (MedCalc Software, Ostend, Belgium; https://www.medcalc.org; 2020), Open Meta analyst (available online at http://www.cebm.brown.edu/openmeta/downloads/open_meta_analyst_win8.zip)[17], and MetaDisc v. 1.4 (available at http://www.hrc.es/investigacion/metadisc_en.htm)[18].
The I2 statistic was used to measure the degree of inconsistency across studies, with I2 values of 25%, 50%, and 75% representing low, moderate, and high substantial heterogeneity. Interpretation of heterogeneity was carried out at a significance level of P = 0.05. The choice of fixed or random effects model was made on the basis of the degree of inconsistency, selecting the random effects model in the case of moderate and high substantial heterogeneity.
The DR of 18F-FDG PET/CT for hematogenous metastases was calculated for per-patient and per-lesion analyses and presented using forest plots.
Diagnostic accuracy (AC) was calculated only for the articles from which it was possible to extract the number of TP, FN, FP, and TN cases on a per-patient and/or per-lesion basis. AC was presented using summary receiver operating curve (SROC).
Significant difference between accuracy of 18F-FDG PET/CT and other imaging modalities was assessed, using a significance threshold of 0.05.
Results
Literature search and eligibility assessment
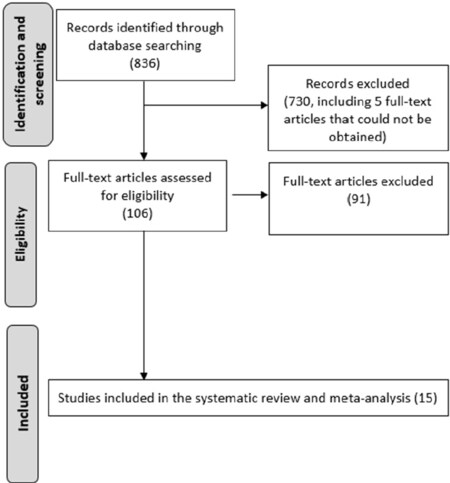
The comprehensive computer literature search from PubMed/MEDLINE database revealed 836 articles [Figure 1]. Reviewing titles and abstracts, 725 articles were excluded. In particular, 725 were not in the field of interest of the present study or were non-original articles. Five further articles were excluded because the full-text could not be obtained despite contacting the authors.
The full text of the remaining 106 studies was evaluated to assess the inclusion criteria. After checking the full-text, 91 articles were excluded, since at least one of the inclusion criteria was not met. No additional records were retrieved after crosschecking the references.
Ultimately, the literature search led to 15 studies suitable for the inclusion in the systematic review and meta-analysis[19-31].
Qualitative analysis (systematic review)
The characteristics of the 15 selected studies are presented in Table 1. All these articles were single-center investigations, published within the last 10 years, except one (2007), by authors from Europe (n = 8) and Asia (n = 7). Two thirds of the studies were retrospective and one third were prospective.
Main characteristics of studies with patients with thyroid cancer and hematogenous metastases evaluated by 18F-FDG PET/CT
| Authors | Years | No. patients | Histotype | Other imaging modalities used for comparison | Standard of reference |
|---|---|---|---|---|---|
| Freudenberg et al.[19] | 2007 | 36 | Papillary (21), Follicular (15) | CT | Histology, clinical follow-up |
| Klain et al.[20] | 2020 | 40 | Differentiated (papillary = 30) | MRI, 18F-FDG PET/MRI | Clinical follow-up |
| Kundu et al.[21] | 2015 | 62 | Papillary (56), Follicular (6) | 68Ga-DOTANOC PET/CT | Histology, clinical follow-up |
| Leboulleux et al.[22] | 2012 | 34 | Papillary (32), Follicular (2), Poorly differentiated (2) | CT, 131I-WBS | Clinical follow-up |
| Nagamachi et al.[23] | 2011 | 70 | Papillary (62), Follicular (8) | MRI, 131I-WBS | Histology, clinical follow-up |
| Nakajo et al.[24] | 2013 | 20 | Papillary (19), Follicular (1) | 18F-FLT PET/CT | Clinical follow-up |
| Ota et al.[25] | 2013 | 11 | Papillary (4), Follicular (7) | Bone scan (planar and SPECT), 18F-fluoride PET/CT | Clinical follow-up |
| Piccardo et al.[26] | 2011 | 20 | Papillary (12), Follicular (8) | 131I-WBS, C.I. | Clinical follow-up |
| Piccardo et al.[27] | 2019 | 25 | Papillary (18), Follicular (7) | 18F-choline PET/CT | Histology, clinical follow-up |
| Poisson et al.[28] | 2010 | 20 | Anaplastic | CT | Clinical follow-up |
| Qiu et al.[29] | 2012 | 80 | Papillary (37), Follicular (39), Follicular variant of papillary (4) | 131I-SPECT/CT, bone scan | Histology, clinical follow-up |
| Sakurai et al.[30] | 2012 | 23 | Papillary (6), Follicular (17) | MRI | Clinical follow-up |
| Shinto et al.[31] | 2015 | 28 | Papillary (10), Follicular (14), Hurtle cell (4) | 99mTc-Hynic TOC SPECT/CT | Clinical follow-up |
| Vrachimis et al.[33] | 2016 | 26 | Differentiated, with suspicion or known dedifferentiation | 18F-FDG PET/MRI, 68Ga-DOTANOC PET/CT | Clinical follow-up |
| Vrachimis et al.[34] | 2016 | 12 | Papillary (5), Follicular (3) Poorly differentiated (4) | 68Ga-DOTATATE PET/MRI, MRI | Histology, clinical follow-up |
The patient sample was consistently relatively small across the studies ranging from 11 to 80. The histotype most represented was papillary (67%), followed by follicular (27%). Only one study recruited exclusively patients with anaplastic histotype.
In the 15 studies, patients underwent 18F-FDG PET/CT scan at different moments of the diagnostic work-up before or after at least one cycle of 131I treatment.
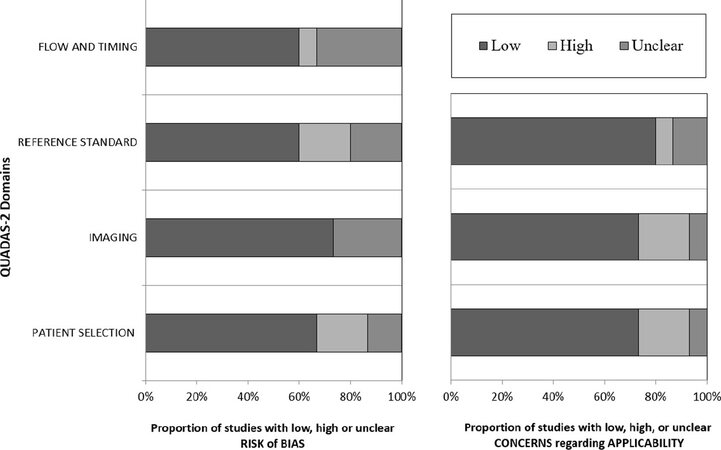
The hematogenous lesions investigated were located prevalently in bone and lung. In each study, PET was compared with at least one imaging modality. In four studies, 18F-FDG PET/CT was assessed in a head-to-head comparison with MRI, prevalently with diffusion-weighted imaging (DWI). CT was available for comparison with 18F-FDG PET/CT in three studies. Patients underwent both PET/MRI and PET/CT in three studies. The standard of reference was based on clinical imaging follow-up in most studies; histology was adopted as additional standard of reference in six studies. The risk of bias for the studies was scored as low by using the QUADAS-2 [Figure 2].
Meta-analysis: detectability of hematogenous metastatic lesions by means of 18F-FDG PET/CT
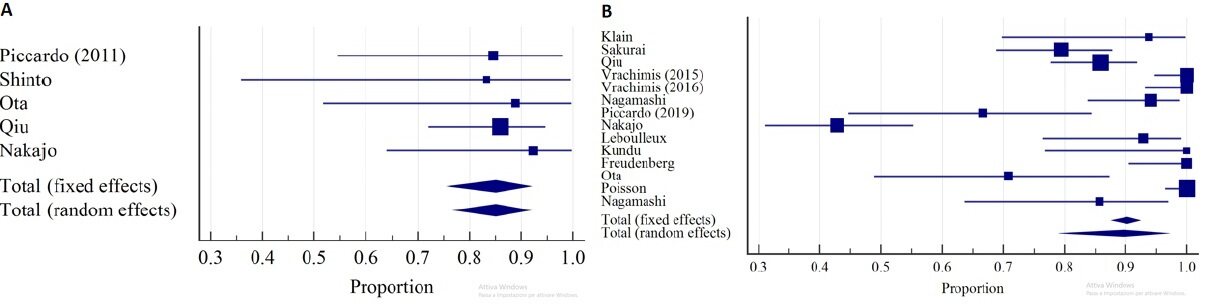
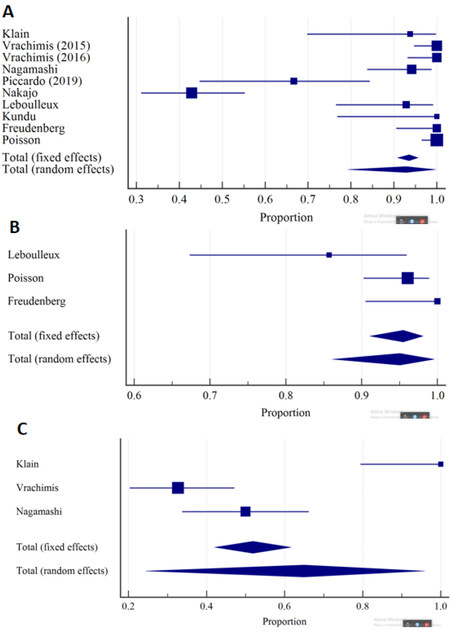
Considering the five studies (patients = 84) for which patient-based analysis was available, 18F-FDG PET/CT detected hematogenous metastases in 85.08% (95% C.I.: 75.96%-91.75%) of cases [Figure 3].
Figure 3. Forest plot of DR of 18F-FDG PET/CT for hematogenous metastases in: per-patient analysis (A); and per-lesion analysis (B).
Considering the 691 lesions (14 studies), 18F-FDG PET/CT showed a pooled DR of 89.70% (95%CI: 79.61%-96.62%) with substantial heterogeneity (I2 = 92.84%).
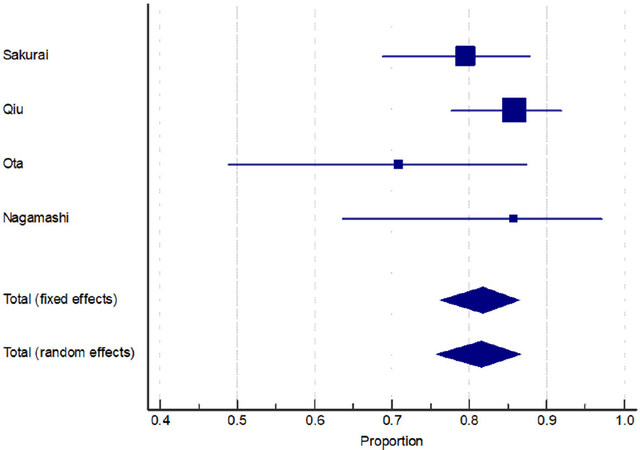
In the sub-group analysis of bone lesions (n = 229; 4 studies), a consistent (I2 = 13.21%) and high DR was found [81.78%; 95%CI: 76.21%-86.52%; Figure 4].
For the sub-group analysis of lung lesions, DR for 18F-FDG PET/CT, CT, and MRI were 92.77%, 95.02%, and 64.93%, respectively [Figure 5]. Whereas a significant difference was found comparing the DR of 18F-FDG PET/CT with that of MRI (P < 0.001), no significant difference was detected between 18F-FDG PET/CT and CT (P = 0.4).
Meta-analysis: accuracy of 18F-FDG PET/CT in detecting hematogenous metastatic lesions
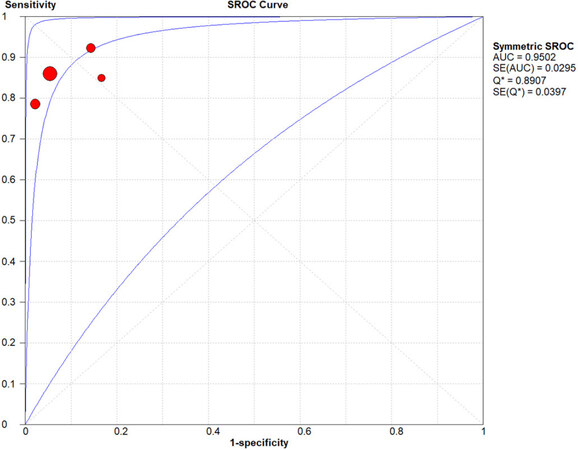
In the per-patient analysis (71 patients with hematogenous metastases out of 139 patients; 4 articles), 18F-FDG PET/CT demonstrated a pooled sensitivity (SS) of 87.3% (95%CI: 77.3%-94%) and a pooled specificity (SP) of 95.6% [95%CI: 87.6-99.1; Figure 6], with nine FN and three FP patients.
Figure 6. Summary receiver operating characteristic curve for detection of hematogenous metastases by 18F-FDG PET/CT in the patient-based analysis.
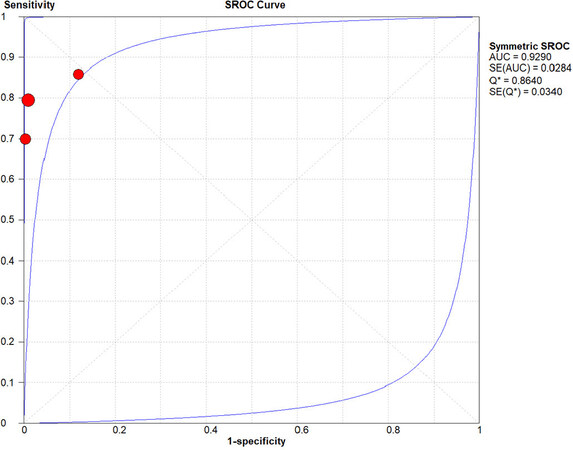
In the per-lesion analysis (including 290 metastases out of 1038 suspicious lesions; 5 studies), PET/CT showed a pooled SS of 86.3% (95%CI: 73.5%-93.5%) and SP of 93.4% (95%CI: 71.7%-98.8%) in the detection of hematogenous metastases [Figure 7], with 10 FP and 66 FN findings. Substantial heterogeneity was found for both SS (I2 = 72.04) and SP (I2 = 82.02%).
Figure 7. Summary receiver operating characteristic curve (SROC) for detection of hematogenous metastases by 18F-FDG PET/CT in the per-lesion analysis.
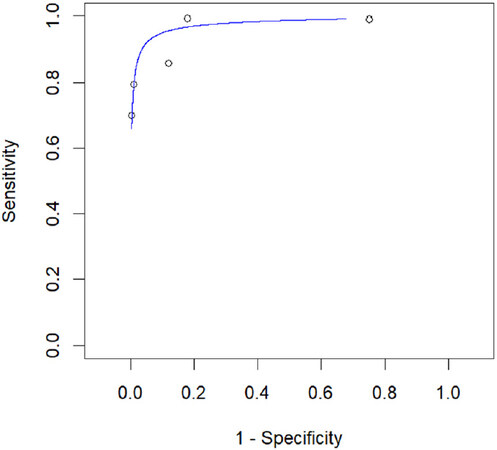
When restricting the analysis to bone metastases (3 articles, with 170 metastatic lesions out of 614 suspicious lesions), corresponding pooled SS was 81.7% (95%CI: 75.8%-86.7%; I2 = 37.9%) and pooled SP was 98.33% (95%CI: 96.5%-99.3%; I2 = 88.5%) [Figure 8]. Only 7 FP and 38 FN findings were found. Per-lung lesion analysis was not performed due to the only 2 articles available.
Discussion
Our meta-analysis demonstrated the utility of 18F-FDG PECT/CT in depicting hematogenous metastases in patients with thyroid cancer. Indeed, the pooled DR for hematogenous metastases was 89.70%. Nevertheless, the substantial heterogeneity points out the small samples of the studies[32].
The location of the lesions probably also influenced the heterogeneity (I2 = 92.84%) of the overall DR, as, in the subgroup analysis of bone lesions, the variability of DR across the studies was negligible (I2 = 13.21%). These data may also indicate a high inter-subject repeatability of 18F-FDG PECT/CT in depicting lesions suspicious for bone metastases in patients with thyroid cancer. Although MRI, with the inclusion of DWI, may assist in the differentiation of cellularity and is generally advocated in the case of vertebral metastases, Sakurai et al.[30] demonstrated a similar DR of MRI compared to 18F-FDG PECT/CT, whereas Nagamachi and colleagues documented a lower detectability of lesions by means of MRI in a patient-based analysis compared to 18F-FDG PECT/CT (84.2% vs. 57.6%)[23]. In our meta-analysis, the three available articles[25,29,30] documented a SS of 71%, 86%, and 79% and corresponding SP of 100%%, 88%, and 99% for bone lesions for 18F-FDG PECT/CT. Interestingly, Ota et al.[25] proved 18F-Fluoride PET/CT as a potential optimal alternative to 18F-FDG PECT/CT for the detection of bone metastases from thyroid cancer, as in their study 18F-Fluoride PET/CT demonstrated higher SS and SP compared to 18F-FDG PECT/CT.
For lung lesions, it was possible to compare the DR of 18F-FDG PECT/CT with that of CT. As expected, CT did not perform inferiorly compared to 18F-FDG PECT/CT. Nevertheless, 18F-FDG PECT/CT proved superior to MRI in this setting (95.02% vs. 64.93%). The sensitivity of PECT/CT in the detection of lung metastases, with corresponding TP and FN cases, was reported in only 2 studies by Vrachimis (100%, 52 lung metastases[33]; 100%, 68 lung metastases[34]). Nakajo et al.[24] reported in their study that FN lung lesions at FDG PET imaging were significantly smaller than TP lung lesions (mean size = 3.8 ± 2.1 mm vs. 9.5 ± 6.2 mm; P = 0.001).
When assessing the diagnostic AC of 18F-FDG PECT/CT, it appeared clear that a further advantage of 18F-FDG PECT/CT in the detection of hematogenous metastases is its very high specificity (95.6% on a per-patient basis and 93.4% on a per-lesion basis). Unfortunately, due to the available literature, no comparison with the diagnostic AC of other imaging techniques was possible. In addition, for the diagnostic AC, the substantial heterogeneity of findings is attributable mainly to the small samples of the studies.
The diagnostic performance of 131I-WBS (planar) was consistently reported lower compared to 18F-FDG PECT/CT in the detection of hematogenous metastases. Indeed, the study of Nagamachi et al.[23] showed a lower DR for 131I-WBS compared to 18F-FDG PECT/CT (71.43% vs. 85.71%); similarly, the performance of 131I-WBS has proved substantially inferior to that of 18F-FDG PECT/CT in the detection of lung metastases[22,23]. Differently, 131I-WBS SPECT/CT may provide a gain in the detection of bone metastases compared to planar imaging. Indeed, in the study of Qiu et al.[29], the accuracy of 131I-WBS SPECT/CT was 93.92%, whereas the accuracy of 18F-FDG PECT/CT was 86.49%. Unfortunately, no other study in the literature provides a head-to-head comparison of 131I-WBS SPECT/CT and 18F-FDG PECT/CT in the depiction of bone metastases.
Other investigated imaging techniques that have been compared to 18F-FDG PECT/CT for the detection of hematogenous metastases include 18F-FDG PET/MRI and PET imaging with 68Ga DOTA derivatives[20,33,34]. In the study of Klain et al.[20], the use of 18F-FDG PET/MRI did not result in additional benefit in the assessment of lung metastases, obtaining a lower SS (87.5%) compared to 18F-FDG PET/CT (93.75%) and MRI (100%). Likewise, 18F-FDG PET/MRI was outperformed by 18F-FDG PET/CT in the detection of lung metastases in the study of Vrachimis et al.[34] (AC: 79.01% vs. 97.53%)[33]. According to the limited available literature, the use of 68Ga DOTA derivatives does not seem to provide noticeable advantages in terms of diagnostic accuracy for lung lesions.
Our meta-analysis presented some limitations. The selected studies provided small sample sizes. Due to the mixed population of most studies, a subgroup analysis of the performance of 18F-FDG PET/CT based on the histological type was not possible. We also noticed the impossibility to extract accurate data on the diagnostic performance of 18F-FDG PET/CT for subjects with unelevated Tg and/or positive 131I-WBS from the included studies in the present meta-analysis; it is conceivable that the diagnostic performance of PET/CT would be inferior for this subgroup of patients compared to the general population.
A source of bias may derive from the substantially high heterogeneity of findings across the studies. Further sources of bias may arise from methodological differences across the studies, including the type of PET/CT scanner. Given the wide range of publication date (2007-2020), studies used PET scanners with different scintillating crystals and reconstruction methods. Furthermore, we noticed that no study using PET/CT scanners equipped with a solid-state detector was found in our meta-analysis. It would be interesting in the future to assess whether these PET/CT scanners may provide a better diagnostic performance in detecting metastases in patients with advanced DTC.
Conclusion
The present meta-analysis suggests the use of 18F-FDG PECT/CT for the identification of hematogenous metastases in patients with thyroid cancer. Nevertheless, the limited number of available studies urges further research in this setting. Comparative studies are still needed to clarify the performance of 18F-FDG against other radiotracers and imaging techniques. Furthermore, studies evaluating the impact of diagnostic performance of 18F-FDG PET/CT on patient management are warranted.
Declarations
Authors’ contributionsPerformed data search, statistical analysis and wrote the first draft of the manuscript: Quartuccio N.
Made substantial contributions to conception and design of the study and revision: Rubello D.
Provided technical and editing support: Rubello D.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2021.
REFERENCES
1. Asa SL. The current histologic classification of thyroid cancer. Endocrinol Metab Clin North Am 2019;48:1-22.
2. Giovanella L. Thyroglobulin measurement in differentiated thyroid carcinoma management. Expert Rev Endocrinol Metab 2008;3:117-25.
3. Raue F, Frank-raue K. Long-Term Follow-up in Medullary Thyroid Carcinoma. In: Raue F, editor. Medullary Thyroid Carcinoma. Cham: Springer International Publishing; 2015. pp. 207-25.
4. Nervo A, Ragni A, Retta F, et al. Bone metastases from differentiated thyroid carcinoma: current knowledge and open issues. J Endocrinol Invest 2021;44:403-19.
5. Phay JE, Ringel MD. Metastatic mechanisms in follicular cell-derived thyroid cancer. Endocr Relat Cancer 2013;20:R307-19.
6. Pacini F, Ito Y, Luster M, Pitoia F, Robinson B, Wirth L. Radioactive iodine-refractory differentiated thyroid cancer: unmet needs and future directions. Expert Rev Endocrinol Metab 2012;7:541-54.
7. Marcus C, Whitworth PW, Surasi DS, Pai SI, Subramaniam RM. PET/CT in the management of thyroid cancers. AJR Am J Roentgenol 2014;202:1316-29.
8. Nanni C, Rubello D, Fanti S, et al. Role of 18F-FDG-PET and PET/CT imaging in thyroid cancer. Biomed Pharmacother 2006;60:409-13.
9. Treglia G, Annunziata S, Muoio B, Salvatori M, Ceriani L, Giovanella L. The role of fluorine-18-fluorodeoxyglucose positron emission tomography in aggressive histological subtypes of thyroid cancer: an overview. Int J Endocrinol 2013;2013:856189.
10. Dong MJ, Liu ZF, Zhao K, et al. Value of 18F-FDG-PET/PET-CT in differentiated thyroid carcinoma with radioiodine-negative whole-body scan: a meta-analysis. Nucl Med Commun 2009;30:639-50.
11. Kim SJ, Lee SW, Pak K, Shim SR. Diagnostic performance of PET in thyroid cancer with elevated anti-Tg Ab. Endocr Relat Cancer 2018;25:643-52.
12. Qichang W, Lin B, Gege Z, et al. Diagnostic performance of 18F-FDG-PET/CT in DTC patients with thyroglobulin elevation and negative iodine scintigraphy: a meta-analysis. Eur J Endocrinol 2019;181:93-102.
13. Lee DY, Kim YI. Peptide Receptor Radionuclide Therapy in Patients With Differentiated Thyroid Cancer: A Meta-analysis. Clin Nucl Med 2020;45:604-10.
14. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097.
15. Campbell I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med 2007;26:3661-75.
16. Whiting PF, Rutjes AW, Westwood ME, et al; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36.
17. Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. J Stat Soft 2012;49.
18. Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;6:31.
19. Freudenberg LS, Frilling A, Kühl H, et al. Dual-modality FDG-PET/CT in follow-up of patients with recurrent iodine-negative differentiated thyroid cancer. Eur Radiol 2007;17:3139-47.
20. Klain M, Nappi C, Nicolai E, et al. Comparison of simultaneous 18F-2-[18F] FDG PET/MR and PET/CT in the follow-up of patients with differentiated thyroid cancer. Eur J Nucl Med Mol Imaging 2020;47:3066-73.
21. Kundu P, Lata S, Sharma P, Singh H, Malhotra A, Bal C. Prospective evaluation of (68)Ga-DOTANOC PET-CT in differentiated thyroid cancer patients with raised thyroglobulin and negative (131)I-whole body scan: comparison with (18)F-FDG PET-CT. Eur J Nucl Med Mol Imaging 2014;41:1354-62.
22. Leboulleux S, El Bez I, Borget I, et al. Postradioiodine treatment whole-body scan in the era of 18-fluorodeoxyglucose positron emission tomography for differentiated thyroid carcinoma with elevated serum thyroglobulin levels. Thyroid 2012;22:832-8.
23. Nagamachi S, Wakamatsu H, Kiyohara S, et al. Comparison of diagnostic and prognostic capabilities of 18F-FDG-PET/CT, ¹³¹I-scintigraphy, and diffusion-weighted magnetic resonance imaging for postoperative thyroid cancer. Jpn J Radiol 2011;29:413-22.
24. Nakajo M, Nakajo M, Jinguji M, et al. Diagnosis of metastases from postoperative differentiated thyroid cancer: comparison between FDG and FLT PET/CT studies. Radiology 2013;267:891-901.
25. Ota N, Kato K, Iwano S, et al. Comparison of 18F-fluoride PET/CT, 18F-FDG PET/CT and bone scintigraphy (planar and SPECT) in detection of bone metastases of differentiated thyroid cancer: a pilot study. Br J Radiol 2014;87:20130444.
26. Piccardo A, Foppiani L, Morbelli S, Bianchi P, Barbera F, Biscaldi E, Altrinetti V, Villavecchia G, Cabria M. Could [18]F-fluorodeoxyglucose PET/CT change the therapeutic management of stage IV thyroid cancer with positive (131)I whole body scan? Q J Nucl Med Mol Imaging 2011;55:57-65.
27. Piccardo A, Trimboli P, Puntoni M, et al. Role of 18F-Choline Positron Emission Tomography/Computed Tomography to Detect Structural Relapse in High-Risk Differentiated Thyroid Cancer Patients. Thyroid 2019;29:549-56.
28. Poisson T, Deandreis D, Leboulleux S, et al. 18F-fluorodeoxyglucose positron emission tomography and computed tomography in anaplastic thyroid cancer. Eur J Nucl Med Mol Imaging 2010;37:2277-85.
29. Qiu ZL, Xue YL, Song HJ, Luo QY. Comparison of the diagnostic and prognostic values of 99mTc-MDP-planar bone scintigraphy, 131I-SPECT/CT and 18F-FDG-PET/CT for the detection of bone metastases from differentiated thyroid cancer. Nucl Med Commun 2012;33:1232-42.
30. Sakurai Y, Kawai H, Iwano S, Ito S, Ogawa H, Naganawa S. Supplemental value of diffusion-weighted whole-body imaging with background body signal suppression (DWIBS) technique to whole-body magnetic resonance imaging in detection of bone metastases from thyroid cancer. J Med Imaging Radiat Oncol 2013;57:297-305.
31. Shinto AS, Kamaleshwaran KK, Mallia M, et al. Utility of (99m)Tc-Hynic-TOC in 131I Whole-Body Scan Negative Thyroid Cancer Patients with Elevated Serum Thyroglobulin Levels. World J Nucl Med 2015;14:101-8.
32. IntHout J, Ioannidis JP, Borm GF, Goeman JJ. Small studies are more heterogeneous than large ones: a meta-meta-analysis. J Clin Epidemiol 2015;68:860-9.
33. Vrachimis A, Burg MC, Wenning C, et al. [(18)F]FDG PET/CT outperforms [(18)F]FDG PET/MRI in differentiated thyroid cancer. Eur J Nucl Med Mol Imaging 2016;43:212-20.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Quartuccio N, Rubello D. Role of 18F-FDG PET/CT in detection of hematogenous metastases of advanced differentiated thyroid carcinoma: a systematic review and meta-analysis. J Cancer Metastasis Treat 2021;7:14. http://dx.doi.org/10.20517/2394-4722.2020.118
AMA Style
Quartuccio N, Rubello D. Role of 18F-FDG PET/CT in detection of hematogenous metastases of advanced differentiated thyroid carcinoma: a systematic review and meta-analysis. Journal of Cancer Metastasis and Treatment. 2021; 7: 14. http://dx.doi.org/10.20517/2394-4722.2020.118
Chicago/Turabian Style
Quartuccio, Natale, Domenico Rubello. 2021. "Role of 18F-FDG PET/CT in detection of hematogenous metastases of advanced differentiated thyroid carcinoma: a systematic review and meta-analysis" Journal of Cancer Metastasis and Treatment. 7: 14. http://dx.doi.org/10.20517/2394-4722.2020.118
ACS Style
Quartuccio, N.; Rubello D. Role of 18F-FDG PET/CT in detection of hematogenous metastases of advanced differentiated thyroid carcinoma: a systematic review and meta-analysis. J. Cancer. Metastasis. Treat. 2021, 7, 14. http://dx.doi.org/10.20517/2394-4722.2020.118
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 27 clicks
Cite This Article 27 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.