Immunotherapy as maintenance treatment in metastatic triple negative breast cancer
Abstract
Metastatic triple negative breast cancer (mTNBC) is an aggressive disease associated with a poor prognosis as compared to other subtypes of breast cancer. Significant advances have been made in recent years with new approvals for PARP inhibitors for those patients who harbor germline BRCA mutations and immune checkpoint inhibitors for patients with programmed death ligand-1 (PD-L1) expressing tumors. These therapies are associated with favorable toxicity profiles and improved health-related quality of life when compared with chemotherapy. Maintenance therapy, now recognized as the mainstay for patients with ovarian malignancies, takes advantage of the benefit of low-intensity therapies to suppress a disease over a prolonged period of time following maximal response to induction therapy. This strategy has been little explored in the treatment of mTNBC. Here, we briefly discuss the evidence to date lending credence to this treatment paradigm and examine the potential role of immunotherapy as maintenance in the management of mTNBC.
Keywords
INTRODUCTION
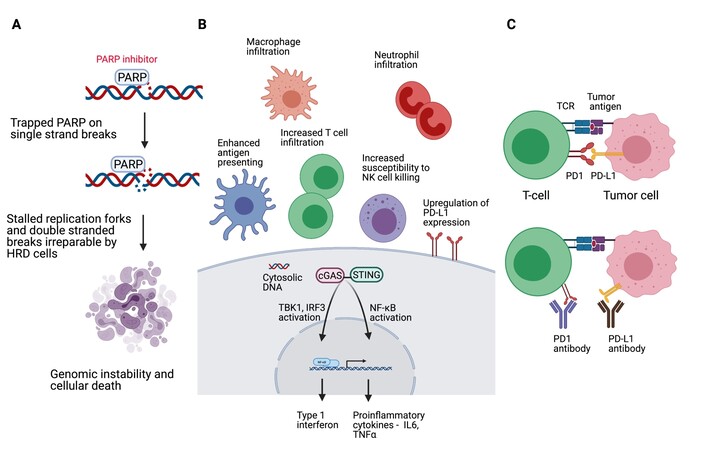
Metastatic triple negative breast cancer (mTNBC) is incurable. An aggressive disease, mTNBC is associated with a poorer prognosis. Until recently, chemotherapy has been the mainstay of treatment with a median overall survival (OS) reported in contemporary chemotherapeutic trials as approximately 12 months[1,2]. In 2018, the United States Food and Drug Administration (FDA) approved the use of the poly (ADP-ribose) polymerase (PARP) inhibitors olaparib and talazoparib for the treatment of human epidermal growth factor receptor-2 (HER2) negative metastatic breast cancers (MBC), which are associated with deleterious germline BRCA mutations[3,4]. The approvals were based on two separate phase 3 randomized controlled studies which evaluated the respective PARP inhibitor against chemotherapy of physician’s choice, which notably excluded platinum agents[3,4]. Consistent absolute improvement in progression free survival (PFS) of approximately three months was noted in both studies, but, more importantly, statistically and clinically significant delay in decline of health-related quality of life was demonstrated in favor of the PARP inhibitor arm[5,6]. It is estimated that approximately 10%-20% of TNBCs are associated with germline BRCA mutations[7-9]. That a group of mTNBC may be successfully treated with an orally available selectively targeted therapeutic agent associated with favorable toxicity profile is a huge win for a disease which has been orphaned for so long. The year 2019 marked another significant milestone in the treatment of mTNBC. Atezolizumab, a programmed death ligand-1 (PD-L1) inhibitor, in combination with nab-paclitaxel was granted accelerated approval by the FDA for use in the treatment of previously untreated biomarker positive mTNBC[10]. The biomarker was PD-L1 expression of any intensity in tumor infiltrating immune cells covering more than 1% of tumor area occupied by tumor cells and associated intratumoral and contiguous peritumoral stroma, as assessed by the Ventana SP142 PD-L1 assay. The approval was granted based on IMpassion 130, a placebo-controlled randomized study which demonstrated improvement in the PFS of mTNBC patients with tumors which express PD-L1, from 4.8 months to 7.4 months [hazard ratio (HR) 0.6; 95% confidence interval (CI): 0.48-0.77, P < 0.0001][10]. Formal comparison of the overall survival (OS) of this cohort of patients could not be made due to the statistical design of the study. However, it is notable that the final OS analyses of the PD-L1 positive mTNBC treated with atezolizumab/nab-paclitaxel demonstrated a median OS of 25.4 months (range 19.6-30.7 months)[11]. Reassuringly, a second phase 3 placebo-controlled randomized study to evaluate upfront programmed cell death protein-1 (PD-1) inhibitor (pembrolizumab) in combination with chemotherapy for the treatment of mTNBC, KEYNOTE 355, also reported an improvement in PFS from 5.6 months to 9.7 months (HR 0.65; 95%CI: 0.49-0.86, P = 0.0012) in the cohort of patients with tumors expressing PD-L1 as defined by a Combined Positive Score of more than 10 using the pharmDx 22C3 assay[12]. For this group of patients, the FDA granted accelerated approval to the combination of pembrolizumab and chemotherapy. Overall survival data, a key co-primary endpoint, are eagerly awaited. Figure 1 is a figural depiction of the mechanism of action of PARP inhibitors and inhibitors of the PD1/PD-L1 axis. Despite significant advances made, the goals of care in the treatment of mTNBC remains to optimize tumor control, prolong survival, and palliate tumor-related symptoms while minimizing treatment-related toxicities. International guidelines generally recommend continuation of each regimen until time of disease progression or unacceptable toxicities[13].
Figure 1. Created with BioRender.com. (A) Mechanism of action of PARP inhibitors. PARP inhibitors trap PARP at the sites of single-strand DNA breaks. Ongoing replication forks clash with PARP-DNA complexes resulting in stalling of the replication forks and generation of double-stranded DNA breaks, which in the context of HRD are repaired through low-fidelity pathways, resulting in genomic instability and cellular death. (B) DNA damage drives inflammatory signaling, stimulating infiltration of innate immune cells such as macrophages and neutrophils, and it can make cells more visible and susceptible to killing by T cells and NK cells. In the context of HRD, PARP inhibitors can upregulate PD-L1 expression and induce cytotoxic T-cell recruitment, which is mediated through cGAS/STING pathway activation. (C) PD-1 signaling negatively regulates T-cell mediated immune response. PD-1 or PD-L1 inhibitors blocks PD-1/PD-L1 interaction, which facilitates an immune response that results in tumor cell kill. Cgas: Cyclic GMP-AMP synthase; HRD: homologous recombination deficiency; IL6: interleukin 6; IRF3: interferon regulatory factor 3; NF-B: nuclear factor-B; NK cell: natural killer cell; PARP: poly(ADP-ribose) polymerase; PD-1: programmed cell death protein 1; PD-L1: programmed death-ligand 1; STING: stimulator of interferon genes; TBK1: TANK-binding kinase 1; TCR: T-cell receptor; TNFα: tumor necrosis factor α.
MAINTENANCE THERAPY
In a systemic review and meta-analysis of chemotherapy duration in the first-line treatment of MBC, longer chemotherapy duration was associated with significantly longer PFS (HR 0.64; 95%CI: 0.55-0.76; P < 0.001) which translated to longer OS (HR 0.91; 95%CI: 0.84-0.99; P = 0.046)[14]. Uninterrupted therapy in the treatment of mTNBC following an effective induction therapy is however often associated with a certain degree of toxicity precluding indefinite continuation of the same regimen. Maintenance therapy is the use of low-intensity therapies to suppress the disease over a prolonged period of time following maximal response to induction therapy. The purpose of maintenance therapy is to prolong the duration of tumor control and therefore life expectancy while maintaining a reasonable quality of life with acceptable adverse effects from treatment. Maintenance therapy can involve either switching to a different compound or continuation of a drug or combinations of drugs of the induction regimen. This is a strategy successfully employed in the treatment of solid organ malignancies such as ovarian, lung, and colorectal cancers[15-17]. In the case of MBC, more specifically in the treatment of HER2-positive MBC, the patient is often treated with 4-6 months of chemotherapy or to time of maximal response, following which HER2-targeted therapy is continued without the chemotherapy[18]. Since publication of the meta-analysis of chemotherapy duration, the only large phase 3 randomized trial to evaluate the role of continuous chemotherapy was conducted by the Korean Cancer Study Group. The authors evaluated maintenance chemotherapy vs. observation in patients with MBC after disease control with six cycles of gemcitabine plus paclitaxel as first-line therapy[19]. The result of this study is an improved PFS (7.5 months vs. 3.8 months, HR 0.73; 95%CI: 0.55-0.97; P = 0.26) and OS (32.3 months vs. 23.5 months; HR 0.65; 95%CI: 0.42-0.99, P = 0.047) in favor of maintenance chemotherapy[19]. Notably, subgroup analyses identified the hormone receptor negative subgroup as more likely to benefit from maintenance chemotherapy (HR 0.52; 95%CI: 0.30-0.90)[19]. Other studies specifically evaluating the question of maintenance treatment in MBC have otherwise been limited to small phase 2 single arm studies [Table 1].
Studies evaluating maintenance therapy for treatment of metastatic breast cancer
| Trial | Trial patient | Study phase (N) | Induction therapy | Maintenance therapy | PFS (months) | OS (months) |
| KCSG-BR07-02 Park et al.[20] 2013 | HER2-negative MBC First-line | Phase 3 324 | Paclitaxel 175 mg/m2 + Gemcitabine 1250 mg/m2 days 1, 8 q21d × 6 cycles | Paclitaxel 175 mg/m2 + Gemcitabine 1250 mg/m2 days 1, 8 q21d vs. observation | Maintenance: 7.5 Observation: 3.8 HR 0.73, 95%CI: 0.55-0.97, P = 0.026 | Maintenance: 32.3 Observation: 23.5 HR 0.65, 95%CI: 0.42-0.99, P = 0.047 |
| Surmeli et al.[20] 2015 | HER2-negative MBC First-line | Phase 2 55 | Docetaxel 75 mg/m2 day 1 + Capecitabine 1650 mg/m2/day days 1-14 q21d × 6 cycles | Capecitabine 2000 mg/m2/day days 1-14 q21d | 9.8 95%CI: 8.4-11.2 | 26.6 95%CI: 21.8-30.1 |
| GINECO A-TaXel Ferrero et al.[21] 2016 | mTNBC First-line | Phase 2 62 | Paclitaxel 80 mg/m2 days 1, 8, 15 + Capecitabine 1600 mg/m2/day days 1-5, 8-12, 15-19 + Bevacizumab 10mg/kg day 1, 15 × 6 q28d cycles | Capecitabine 1600 mg/m2/day days 1-5, 8-12, 15-19 + Bevacizumab 10mg/kg day 1, 15 q28d | 7.6 95%CI: 6.3-9.0 | 19.2 95%CI: 17.4-20.9 |
| IBCSG 42-12/BIG 2-12 SNAP trial Gennari et al.[22] 2018 | HER2-negative MBC First-line | Phase 2 255 | Nab-paclitaxel 150/125 mg/m2 days 1, 8, 15 q28d × 3 cycles | Arm A: Nab-paclitaxel 150 mg/m2 days 1, 15 q28d Arm B: Nab-paclitaxel 100 mg/m2 days 1, 8, 15 q28d Arm C: Nab-paclitaxel 75 mg/m2 days 1, 8, 15, 22 q28d | Arm A: 7.9 90%CI: 6.8-8.4 Arm B: 9 90%CI: 8.1-10.9 Arm C: 8.5 90%CI: 6.7-9.5 | NR |
| SBCCSG 35 Inoue et al. [23] 2018 | HER2-negative MBC < 3 lines chemotherapy | Phase 2 51 | Paclitaxel 90 mg/m2 days 1, 8, 15 + Bevacizumab 10mg/kg days 1, 15 q28d × 3 cycles | Eribulin 1.4 mg/m2 days 1, 8 q21d | 10.7 95%CI: 9.6-11.8 | 20.0 95%CI: 16.0-24.0 |
| Symonds et al. [24] 2019 | mTNBC First-line | Phase 2 55 | Nab-paclitaxel 100 mg/m2 days 1, 8, 15 + Bevacizumab 10mg/kg days 1, 15 q28d × 6 cycles | Erlotinib 150mg/day + Bevacizumab 10mg/kg days 1, 15 q28d | 9.1 95%CI: 7.2-11.1 | 18.1 95%CI: 15.6-21.7 |
More recently, two studies have reported interesting results to support maintenance with an alternate therapy to chemotherapy. BROCADE3 is a randomized placebo-controlled phase 3 trial which evaluated the combination of platinum chemotherapy and the PARP inhibitor veliparib for the treatment of BRCA mutation associated HER2-negative MBC[25]. In total 513 patients, approximately half of whom had mTNBC, were randomly assigned to paclitaxel and carboplatin with veliparib or the same chemotherapy with placebo. Patients in both groups received similar duration of chemotherapy of approximately 11 cycles, following which 41% of patients in the veliparib group and 34% of patients in the control group discontinued chemotherapy to receive continuous single-agent veliparib at higher doses of 300 or 400 mg twice daily[25]. The mean overall duration of blinded monotherapy in these patients was 350 days [standard deviation (SD) 318 days] in the veliparib group versus 252 days (SD 263 days) in the control group[25]. More patients were alive and progression free in the veliparib group than in the control group at three years [25.7% (95%CI: 20.3-31.4%) vs. 10.7% (95%CI: 5.8-17.3%)], lending support to an intriguing question of the use of PARP inhibitors as maintenance[25]. The SAFIR02-BREAST IMMUNO sub-study is a phase 2 randomized study which included patients with HER2-negative MBC whose disease did not progress following 6-8 cycles of chemotherapy. Patients were randomized to either maintenance durvalumab, a PD-L1 inhibitor, or maintenance chemotherapy[26]. There was no apparent benefit in terms of PFS in the overall population with switch to durvalumab as compared with chemotherapy (2.7 months vs. 4.6 months, HR 1.4; 95%CI: 1.00-1.96; P = 0.047)[26]. Exploratory analyses however revealed longer OS in favor of maintenance immunotherapy in the TNBC (n = 82, 14.0 vs. 21.2 months, unadjusted HR 0.54; 95%CI: 0.30-0.97; P = 0.04) or PD-L1 positive (n = 44, 12.1 vs. 25.8 months, unadjusted HR 0.42; 95%CI: 0.17-1.05; P = 0.06) subgroup of patients[26].
RATIONALE FOR MAINTENANCE IMMUNOTHERAPY
Apart from having a safety profile which is generally more favorable as compared to chemotherapy, there are several lines of evidence to support the sequencing of immunotherapy as maintenance treatment following chemotherapy induction. Firstly, chemotherapy can promote tumor immunity through induction of immunogenic cell death (ICD). ICD is a form of regulated cellular demise which drives an inflammatory response culminating with activation of cytotoxic T lymphocytes and an adaptive immune response[27]. Conventional chemotherapeutics commonly used in the treatment of MBC which are associated with ICD include anthracyclines, cyclophosphamide, and taxanes[27]. Secondly, chemotherapy can disrupt tumor evasion of the immune system through depletion of suppressive immune cell population. Cyclophosphamide has been demonstrated to deplete regulatory T cells, cisplatin can upregulate major histocompatibility complex class I expression, and doxorubicin is associated with myeloid-derived suppressive cellular depletion and an increase in level of type I interferons[28,29]. Finally, sequencing multiple distinct treatment modalities such as immunotherapy following chemotherapy can potentially achieve higher rates of disease control through non-cross resistance and an increase in likelihood that patients receive potentially active therapy earlier, preventing the emergence of resistant clones.
Ongoing trials evaluating immunotherapy given either alone or in combination as maintenance treatment are shown in Table 2. The combination of PARP inhibitor and immunotherapy is noteworthy because PARP inhibitors have been shown to induce CD8+ T cell infiltration and activation in in vivo models through activation of the cGAS/STING pathway[30,31]. Conceptually, it makes sense to combine these two generally well-tolerated agents without overlapping toxicities and with distinct and potentially complementary mechanisms of actions to further enhance anti-tumor efficacy.
Trials evaluating immunotherapy as maintenance strategy
| Trial/clinicaltrials.gov identifier | Phase, Estimated enrolment | Patient population | Induction | Maintenance |
| NCT02411656 | 2 n = 35 | Metastatic/recurrent inflammatory breast cancer or TNBC | 2 months of chemotherapy not otherwise specified | Pembrolizumab 200 mg q21d |
| DORA NCT03167619 | 2 n = 60 | Metastatic ER low/HER2 negative or TNBC First- or Second-line | 6 × weekly doses or 3 × 3 weekly doses of platinum-based chemotherapy | Olaparib 300mg twice daily or Olaparib 300 mg twice daily + Durvalumab 1500 mg q28d |
| KEYLYNK-009 NCT04191135 | 3 n = 932 | Metastatic TNBC | Carboplatin AUC 2 + Gemcitabine 1000 mg/m2 days 1 and 8 + Pembrolizumab 200 mg q21d × 4-6 cycles | Carboplatin AUC 2 + Gemcitabine 1000 mg/m2 days 1 and 8 + Pembrolizumab 200 mg q21d vs. Pembrolizumab 200 mg q21d + Olaparib 300 mg twice daily |
CONCLUDING WITH MORE UNANSWERED QUESTIONS
Significant strides have been made in the treatment of mTNBC. There are still several unanswered questions regarding optimal sequencing and treatment. Compelling evidence suggests that timing is important to fully maximize the therapeutic potential of immunotherapy. Existing data support immunotherapy in the frontline setting. However, what is the role for retreatment with immunotherapy in the metastatic setting with emerging data heralding chemo-immunotherapy combinations in the (neo)adjuvant treatment of non-metastatic TNBC? Can we find a role for immunotherapy in PD-L1 negative mTNBC? Is immunotherapy best sequenced concomitantly with chemotherapy or following induction chemotherapy? Can we select for patients for whom chemotherapy may be spared? With the ability to unlock long-lasting immunological memory, is there a role for treatment break following maximal response? What is the optimal duration? Will the tumor remain sensitive and amenable to rechallenge following cessation of treatment? We are still in the preliminary stages of clinical investigation. Clearly, more work is required to define the role of maintenance immune checkpoint inhibitors in mTNBC. Survival benefit will have to be critically examined, taking into account cost-effectiveness and the impact upon quality of life.
DECLARATIONS
Authors’ contributionsAll authors contributed to drafting and critical revision of the manuscript.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestTan TJ declares advisory board and speakers honoraria from Roche, Novartis, Astra Zeneca, Pfizer, DKSH, A. Baur & Co., travel support from Eisai and Astra Zeneca as well as grant funding of research from Astra Zeneca. Chan JJ declares advisory board and speakers honoraria from GlaxoSmithKline, AstraZeneca, Pfizer, Merck Sharp & Dohme and Eisai, travel support from AstraZeneca and Pfizer as well as grant funding of research from OncoQuest. Dent RA declares advisory board and speakers honoraria from AstraZeneca, DKSH, Eisai, MSD, Pfizer, Novartis and Roche, travel support from AstraZeneca, Eisai and Pfizer as well as grant funding of research from Astra Zeneca.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2021.
REFERENCES
1. Tutt A, Tovey H, Cheang MCU, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med 2018;24:628-37.
2. O'Shaughnessy J, Schwartzberg L, Danso MA, et al. Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol 2014;32:3840-7.
3. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 2017;377:523-33.
4. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 2018;379:753-63.
5. Robson M, Ruddy KJ, Im SA, et al. Patient-reported outcomes in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer receiving olaparib versus chemotherapy in the OlympiAD trial. Eur J Cancer 2019;120:20-30.
6. Ettl J, Quek RGW, Lee KH, et al. Quality of life with talazoparib versus physician's choice of chemotherapy in patients with advanced breast cancer and germline BRCA1/2 mutation: patient-reported outcomes from the EMBRACA phase III trial. Ann Oncol 2018;29:1939-47.
7. Wong-Brown MW, Meldrum CJ, Carpenter JE, et al. Prevalence of BRCA1 and BRCA2 germline mutations in patients with triple-negative breast cancer. Breast Cancer Res Treat 2015;150:71-80.
8. Gonzalez-Angulo AM, Timms KM, Liu S, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res 2011;17:1082-9.
9. Hartman AR, Kaldate RR, Sailer LM, et al. Prevalence of BRCA mutations in an unselected population of triple-negative breast cancer. Cancer 2012;118:2787-95.
10. Schmid P, Adams S, Rugo HS, et al. IMpassion130 Trial Investigators. Atezolizumab and Nab-Paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108-21.
11. Emens L, Adams S, Barrios C, et al. LBA16 IMpassion130: Final OS analysis from the pivotal phase III study of atezolizumab + nab-paclitaxel vs placebo + nab-paclitaxel in previously untreated locally advanced or metastatic triple-negative breast cancer. Annals of Oncology 2020;31:S1148.
12. Cortes J, Cescon DW, Rugo HS, et al. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J Clin Oncol 2020;38:1000.
13. Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020;31:1623-49.
14. Gennari A, Stockler M, Puntoni M, et al. . Duration of chemotherapy for metastatic breast cancer: a systematic review and meta-analysis of randomized clinical trials. J Clin Oncol 2011;29:2144-9.
15. Walsh CS. Latest clinical evidence of maintenance therapy in ovarian cancer. Curr Opin Obstet Gynecol 2020;32:15-21.
16. Coate LE, Shepherd FA. Maintenance therapy in advanced non-small cell lung cancer: evolution, tolerability and outcomes. Ther Adv Med Oncol 2011;3:139-57.
17. Sonbol MB, Mountjoy LJ, Firwana B, et al. The Role of maintenance strategies in metastatic colorectal cancer: a systematic review and network meta-analysis of randomized clinical trials. JAMA Oncol 2020;6:e194489.
18. Giordano SH, Temin S, Chandarlapaty S, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO clinical practice guideline update. J Clin Oncol 2018;36:2736-40.
19. Park YH, Jung KH, Im SA, et al. Phase III, multicenter, randomized trial of maintenance chemotherapy versus observation in patients with metastatic breast cancer after achieving disease control with six cycles of gemcitabine plus paclitaxel as first-line chemotherapy: KCSG-BR07-02. J Clin Oncol 2013;31:1732-9.
20. Surmeli ZG, Varol U, Cakar B, et al. Capecitabine maintenance therapy following docetaxel/capecitabine combination treatment in patients with metastatic breast cancer. Oncol Lett 2015;10:2598-602.
21. Ferrero JM, Hardy-Bessard AC, Capitain O, et al. Weekly paclitaxel, capecitabine, and bevacizumab with maintenance capecitabine and bevacizumab as first-line therapy for triple-negative, metastatic, or locally advanced breast cancer: results from the GINECO A-TaXel phase 2 study. Cancer 2016;122:3119-26.
22. Gennari A, Sun Z, Hasler-Strub U, et al. A randomized phase II study evaluating different maintenance schedules of nab-paclitaxel in the first-line treatment of metastatic breast cancer: final results of the IBCSG 42-12/BIG 2-12 SNAP trial. Ann Oncol 2018;29:661-8.
23. Inoue K, Ninomiya J, Saito T, Kimizuka K, Kurosumi M. Induction therapy with paclitaxel and bevacizumab followed by switch maintenance therapy with eribulin in Japanese patients with HER2-negative metastatic breast cancer: a multicenter, collaborative, open-label, phase II clinical study for the SBCCSG 35 investigators. BMC Cancer 2018;18:671.
24. Symonds L, Linden H, Gadi V, et al. Combined targeted therapies for first-line treatment of metastatic triple negative breast cancer - a phase II trial of weekly Nab-Paclitaxel and Bevacizumab followed by maintenance targeted therapy with Bevacizumab and Erlotinib. Clin Breast Cancer 2019;19:e283-96.
25. Diéras V, Han HS, Kaufman B, et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Oncol 2020;21:1269-82.
26. Bachelot T, Filleron T, Bieche I, et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: the randomized phase II SAFIR02-BREAST IMMUNO trial. Nat Med 2021;27:250-5.
27. Galluzzi L, Vitale I, Warren S, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer 2020;8:e000337.
28. Voorwerk L, Slagter M, Horlings HM, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med 2019;25:920-8.
29. Heinhuis KM, Ros W, Kok M, et al. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol 2019;30:219-35.
30. Chabanon RM, Muirhead G, Krastev DB, et al. . PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. J Clin Invest. 2019;129:1211-1228.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Tan TJ, Chan JJ, Dent RA. Immunotherapy as maintenance treatment in metastatic triple negative breast cancer. J Cancer Metastasis Treat 2021;7:21. http://dx.doi.org/10.20517/2394-4722.2021.20
AMA Style
Tan TJ, Chan JJ, Dent RA. Immunotherapy as maintenance treatment in metastatic triple negative breast cancer. Journal of Cancer Metastasis and Treatment. 2021; 7: 21. http://dx.doi.org/10.20517/2394-4722.2021.20
Chicago/Turabian Style
Tan, Tira J., Jack J. Chan, Rebecca A. Dent. 2021. "Immunotherapy as maintenance treatment in metastatic triple negative breast cancer" Journal of Cancer Metastasis and Treatment. 7: 21. http://dx.doi.org/10.20517/2394-4722.2021.20
ACS Style
Tan, TJ.; Chan JJ.; Dent RA. Immunotherapy as maintenance treatment in metastatic triple negative breast cancer. J. Cancer. Metastasis. Treat. 2021, 7, 21. http://dx.doi.org/10.20517/2394-4722.2021.20
About This Article
Copyright
Data & Comments
Data

 Cite This Article 3 clicks
Cite This Article 3 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.