18F-FDG-PET/CT-guided radiotherapy of cervical lymph nodes in head and neck squamous cell carcinoma
Abstract
The use of positron emission tomography with fluor-18-fluorodeoxyglucose (FDG-PET) in clinical practice for patients with head and neck squamous cell carcinoma (HNSCC) has expanded rapidly, with implications for diagnostic staging, radiotherapy planning, adaptive radiotherapy, and post-therapy evaluation. The implementation of FDG-PET/CT in radiation treatment planning not only has consequences for target volume definition and dose prescription but is also associated with an increased overall survival in patients with HNSCC. FDG-PET/CT-guided gradient dose prescription provides a window of opportunity for treatment de-intensification of the neck in order to decrease treatment-related toxicity without compromising oncological outcome. Further, interim FDG-PET/CT during radiotherapy can be useful to assess metabolic tumor response and enables opportunities for adaptive treatment strategies. The goals are to increase treatment effectivity in poor responders and reduce unnecessary toxicity in patients with good early tumor response. Further prospective trials investigating adaptive radiotherapy based on interim PET-evaluation are needed, especially regarding human papilloma virus-negative HNSCC and patients treated with primary radiotherapy.
Keywords
INTRODUCTION
In a majority of patients with head and neck squamous cell carcinoma (HNSCC), treatment consists of radiotherapy, and if possible, combined with chemotherapy for locally advanced disease[1-3]. Current dose prescription in curative (chemo)radiotherapy [(C)RT] for HNSCC consists of two dose levels. A high “boost dose”, of around 70 Gy in fractions of 2 Gy (or biologically equivalent: EQD2), is delivered to tumor that can be detected during diagnostic work-up (i.e., macroscopic disease). A lower “elective dose” of
In the past few decades diagnostic imaging techniques have evolved rapidly, contributing to a better nodal staging of the neck in HNSCC. The criterion for pathologic lymph nodes on computed tomography (CT) was limited to a nodal short-axis diameter ≥ 10 mm, because of a rapidly decreasing specificity for smaller nodes[5]. With the use of magnetic resonance imaging (MRI), the detection threshold could be reduced to 7-10 mm, when assessing morphological features such as border irregularity and inhomogeneity[6]. Ultrasound-guided fine needle aspirated cytology (US-FNAC) further improved the detection of smaller lymph node metastases, mainly based on the excellent specificity of pathological examination, but with limited sensitivity and inherent inter-operator variability and practical limitations in the number of evaluable nodes[7,8].
Finally, the introduction of positron emission tomography with fluor-18-fluorodeoxyglucose (FDG-PET) imaging enabled quantitative functional evaluation of lymph nodes in addition to morphologic evaluation. Since the beginning of this century, the use of FDG-PET/CT in clinical practice for patients with HNSCC has expanded rapidly, being far more than only a diagnostic tool for disease staging. This review discusses the role of FDG-PET/CT in radiotherapy of the neck in HNSCC, with implications for diagnostic staging, dose prescription, adaptive radiotherapy, and post-therapy evaluation.
FDG-PET/CT FOR DIAGNOSTIC EVALUATION, CURRENT PRACTICE
Pre-treatment nodal staging
FDG-PET has the unique ability to perform a quantitative functional evaluation of tissues, as FDG-uptake reflects the metabolic activity of tumor cells and can be considered as a surrogate for tumor burden[9]. Especially in HNSCC, FDG-PET/CT is commonly used for diagnostic evaluation of lymph nodes. Several large meta-analyses demonstrate a superior accuracy of FDG-PET/CT for lymph node assessment, in comparison with stand-alone conventional anatomic imaging[10-13]. There are few studies reporting on the detection threshold of FDG-PET for nodal metastases in HNSCC using histopathological validation.
FDG-PET-guided surveillance of the neck
In addition to pre-treatment staging, FDG-PET/CT plays an important diagnostic role in follow-up after after (C)RT for HNSCC. The main focus of post-therapy FDG-PET is the detection of residual disease in cervical lymph nodes. Multiple studies confirm that FDG-PET/CT has a high negative predictive value (> 93%) in the evaluation of residual nodal disease after (C)RT[17-20]. However, there are indications that post-therapy response evaluation with FDG-PET/CT may be less reliable in human papilloma virus (HPV)-positive HNSCC[19]. FDG-uptake as a result of inflammatory response to irradiation of the neck usually declines within weeks, allowing an accurate evaluation at approximately 10-12 weeks after the end of (C)RT[21]. The PET-NECK trial demonstrated that survival was similar among advanced nodal stage HNSCC patients (N2/N3) who underwent PET/CT-guided surveillance 12 weeks after (C)RT, and those who underwent planned neck dissection. However, PET/CT-guided surveillance resulted in sparing of neck dissection in 80% of patients[22]. Occasionally, increased FDG-uptake in certain areas of the neck may persist for months after (C)RT, without evidence of residual disease[23]. The underlying causes include inflammation or ulceration[18]. Correlation with clinical evaluation, anatomical imaging, histopathological validation, and discussion within a multidisciplinary board are critical to correctly differentiate persistent cancer from non-malignant pathology.
FDG-PET/CT-GUIDED RADIOTHERAPY, A NEW ERA
Impact on outcome
The better identification of lymph node metastases by FDG-PET not only has consequences for the radiotherapy target volume, but also has prognostic implications for HNSCC patients treated with (C)RT. Defining the nodal target volume based on FDG-PET/CT results in alteration of nodal radiation treatment in approximately 1 out of 4 patients compared to conventional imaging, with nodal up-staging in 8%-21% and down-staging in 3%-11%[14,24-27]. Recently, van den Bosch et al.[28] described the clinical impact of target volume transformation on radiation treatment outcomes using FDG-PET/CT-based treatment planning. They retrospectively analysed 633 HNSCC patients treated with definitive (C)RT. In 46% of the patients, a diagnostic iodine contrast enhanced FDG-PET/CT in treatment position was acquired for radiotherapy planning. If patients developed a recurrence in the neck after treatment, the exact site of the recurrence was reconstructed by performing co-registration of the diagnostic images showing the recurrence with the initial treatment planning scan. It was demonstrated that FDG-PET/CT-assisted radiation treatment planning is associated with a significantly lower rate of recurrence in the CTVelective-nodal (HR = 0.33; P = 0.026), increased overall regional control (HR = 0.62; P = 0.027), and higher overall survival (HR = 0.71; P = 0.033), compared with CT-only radiotherapy planning[28].
Target volume transformation
Combining FDG-PET/CT with conventional anatomical imaging significantly improves the detection rate of lymph node metastases, which has important consequences for radiotherapy target volume and dose
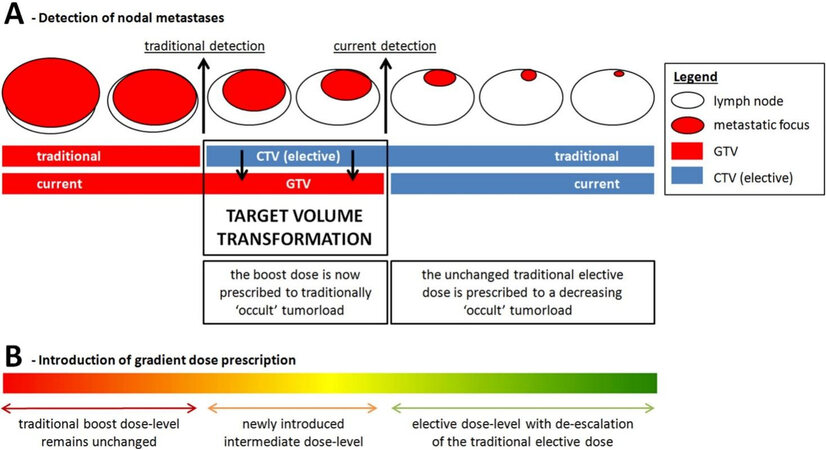
Figure 1. Target volume transformation. Nodal target volume transformation is defined as “upgrading” lymph node metastasis from the elective CTV to GTV based on their increased detectability resulting from improved diagnostic imaging techniques. Target volume transformation may result in overtreatment of both volumes. First, the boost-dose is now prescribed to small lymph node metastases that would have traditionally been treated with the elective dose. Second, the traditional elective dose is prescribed to the elective CTV while the occult tumor volume within the elective CTV is decreased as a result of improved diagnostic imaging (A). By refining traditional binary dose prescription to a gradient dose prescription that is proportional to (occult) tumor volume, the current overtreatment can be addressed in order to decrease treatment-related morbidity without compromising efficacy (B). Reprinted from van den Bosch et al.[29], with permission from Elsevier. CTV: Clinical target volume; GTV: gross tumor volume.
This nodal target volume transformation imposes changes in radiotherapy dose levels that need to be prescribed to these volumes. Moreover, this provides a window of opportunity for treatment de-intensification of the neck in order to decrease treatment-related toxicity without compromising oncological outcome.
Intermediate dose level
First, small nodal metastases that previously remained undetected and used to be part of the CTVelective-nodal will currently be irradiated with a high boost dose of 70 Gy (EQD2) because they are now included in the GTV, which may be unnecessarily high for the relatively low tumor burden in these lymph nodes. Studies investigating recurrence in the electively irradiated neck have identified selection criteria for lymph nodes that can be treated with intermediate dose. An analysis of 1166 electively irradiated lymph nodes in 264 HNSCC patients identified nodal size (summed long- and short-axis diameter ≥ 17 mm) as an important risk factor for nodal failure after elective irradiation with 45 Gy (EQD2)[30]. However, a relevant proportion of nodes with a summed diameter ≥ 17 mm turns out to be false positive, which confirms the need for additional parameters to facilitate adequate risk assessment of lymph nodes[30]. Because FDG-uptake reflects the metabolic activity of tumor deposits it can be used as a surrogate measure of tumor load. FDG-PET/CT has the potential to discriminate between nodes with low, moderate, or high tumor burden using standardized nodal FDG-uptake thresholds[9,31].
By combining nodal size and FDG-uptake as a parameter for tumor load, a nodal risk assessment algorithm for standardized evaluation of lymph nodes could be defined[30-32]. For selected metastases with moderate tumor burden, an intermediate dose level of 60 Gy (EQD2) may be sufficient, as no recurrences in electively irradiated lymph nodes were observed above this dose in the previously mentioned retrospective analysis
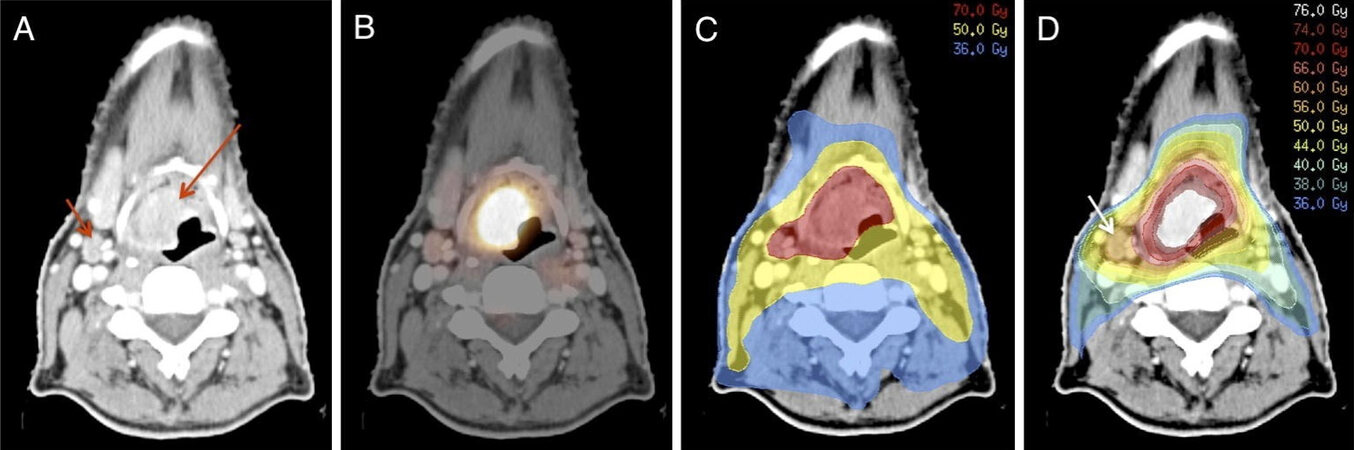
Figure 2. Patient with T3N1M0 squamous cell carcinoma of the supraglottic larynx. CT-scan showing primary tumor (long arrow) and small metastatic node (short arrow) (A). FDG-PET/CT scan showing high FDG-uptake in the primary tumor and moderate uptake in the lymph node indicating low tumor load in this node (B). Conventional radiotherapy dosing; 70 Gy to primary tumor and metastatic node and 50 Gy to elective lymph node regions (C). Gradient radiotherapy dosing; dose escalation (76 Gy) to gross tumor with high FDG-uptake in primary location, 70 Gy to peripheral zone, intermediate dose (60 Gy) to low volume tumor deposit with intermediate FDG-uptake (arrow), and low dose (36-40 Gy) to elective lymph node regions (D). Reprinted from Kaanders et al.[34], with permission from Elsevier. FDG-PET: Fluor-18-fluorodeoxyglucose positron emission tomography.
Elective dose and volume de-escalation
Another consequence of this target volume transformation is that the CTVelective-nodal will contain lower disease volume, while it is nowadays still treated with the unchanged elective dose of 50 Gy (EQD2) originating from more than half a century ago. There is increasing evidence that lymph nodes that contain only very low-volume deposits of metastatic squamous cell carcinoma can be eradicated with doses well below 50 Gy[29,33,35,36].
Deschuymer et al.[35] performed a multicenter randomized controlled trial comparing an equivalent dose of
A single-arm phase 2 study using 36 Gy electively in 54 patients with locally advanced HNSCC showed no elective volume recurrences after a median follow-up of 36 months in surviving patients[39]. Notably, 54% of these patients had HPV-positive oropharyngeal cancer, which is considered to be more radiosensitive and all patients received concurrent chemotherapy. At the Memorial Sloan Kettering Cancer Center, current clinical practice is to de-escalate the dose of the elective neck to 30 Gy (EQD2), albeit only in HPV-positive oropharyngeal cancer receiving concomitant platinum based chemotherapy[40].
Recently the single-arm INFIELD trial investigated the possibility to improve acute and late morbidity of patients with oropharyngeal and laryngeal cancer, both by tailoring the elective irradiation only to regions with a legitimate risk of recurrence (> 5%) and by lowering the elective dose to 40 Gy[36]. Following an “involved node” approach, nodal levels III and IV were only electively irradiated if the immediate proximal level contained pathologic lymph nodes. Irradiation of level IB and V was only performed in case of suspicious lymph nodes in these levels. Ninety percent of the patients received concurrent chemotherapy. At a median follow-up of 24.7 months for surviving patients, there were no solitary recurrences in electively irradiated lymph nodes. Seven of 72 patients developed a nodal recurrence, 5 of which were in-field and 2 occurred in electively irradiated nodes with synchronous in-field recurrence. Patient-reported outcomes assessment at 1 year showed superior or equivalent outcomes compared with baseline, except for saliva and taste measures.
The above findings strongly suggest that the biologically equivalent dose of 36-40 Gy is sufficient to eradicate occult nodal disease after state-of-the-art assessment of the neck. In fact, this dose appears to be sufficient in both radiosensitive non-smoking, HPV-associated oropharyngeal cancer and higher-risk HPV-negative HNSCC. The concept of FDG-PET-guided gradient dose prescription is solely based on the estimation of tumor burden, and de-escalation of the elective dose is performed independent of tumor radiosensitivity. This concept may apply to many other tumor types in which routine treatment includes elective irradiation of nodal areas, such as cancers of the breast, cervix, prostate, rectum and bladder[29]. The effect of dose de-escalation on toxicity may vary according to the anatomical location of target volumes and surrounding organs at risk. However, in all of the aforementioned dose de-escalation studies in HNSCC, a majority of patients received concurrent chemotherapy, which may compensate for a (too) low elective dose because of its radiosensitizing effect. Currently, the ongoing UPGRADE-RT trial (NCT02442375) is the first multicenter randomized controlled trial investigating the safety and efficacy of FDG-PET-guided dose de-escalation in HNSCC patients treated with primary radiotherapy, without the use of concomitant chemotherapy[32]. The primary endpoint of the UPGRADE-RT trial is dysphagia, measured on a 10-step “normalcy of diet” scale from the performance status scale for patients with head and neck cancer. Three-hundred patients will be randomized and accrual is expected to be complete by the end of 2021, and first results to be reported in 2022.
Gradient dose concept
Implementing FDG-PET in radiation treatment planning, enables the opportunity to replace the current two-dose-level practice for a “gradient dose” concept, in which dose is prescribed proportional to tumor burden and the estimated risk of occult disease [Table 1][29]. In the intervention arm of the UPGRADE-RT trial, only a partial implementation of the gradient dose concept is being evaluated[32]. For dose prescription to lymph nodes, an ordinal scale consisting of three dose levels is used, based on a risk assessment algorithm combining nodal size and metabolic activity on FDG-PET/CT as a surrogate for nodal tumor burden. To bring the gradient dose concept further, dose prescription to lymph nodes should ideally be done on a continuous scale, attuned to tumor burden per individual node. The ultimate implementation of this concept would imply selective irradiation of individual lymph nodes harbouring metastases, instead of elective irradiation of complete anatomical nodal levels. There are several innovative diagnostic techniques emerging that could support this implementation, such as ultra-small superparamagnetic iron-oxide nanoparticle (USPIO)-enhanced MRI and sentinel lymph node detection using SPECT/CT[41]. However, the exact diagnostic value of these techniques in the detection of very small nodal tumor deposits is still under investigation, impeding implementation of the gradient dose concept to its full extent at this point.
Overview of current and future concepts in radiotherapy for HNSCC
| Concepts | Currently | Near future | Distant future |
| RT dose/volume | - Traditional binary dose prescription - Large elective irradiation volumes | - FDG-PET guided gradient dose prescription - Sentinel lymph node procedure | - On individual basis, elective volumes will be largely reduced or completely abandoned - Selective irradiation of sentinel lymph node(s) only |
| FDG-PET for radiotherapy planning | - Increasingly used, but not in a systematic manner | - Algorithms for risk assessment of lymph nodes and segmentation of PET-signal | - Biological target volumes based on more specific PET-tracers |
| Interim PET response evaluation | - Not widely implemented in clinical practice | - Dose adaptive RT (e.g., escalation/de-escalation) - Anatomically adaptive RT | - Biological tumor profiling with various PET-tracers to guide patient tailored treatment |
Interim treatment evaluation by PET
Despite treatment with concomitant (C)RT, loco-regional disease relapse occurs within the first 2 years in 30%-50% of patients with locally advanced HNSCC, mostly in initially involved sites[42]. It would be of great help if subgroups of patients that are poor responders to (C)RT can be identified before or early during the treatment. Performing FDG-PET/CT during radiotherapy can be useful to assess metabolic tumor response in addition to volume-based assessment by anatomical imaging. Several studies aimed to identify early prognostic imaging biomarkers using interim FDG-PET, enabling on-treatment decisions to be made regarding modification of treatment strategy (e.g., early dose de-escalation or switch to a different treatment modality)[43-46]. The goals are to increase treatment effectivity in poor responders and reduce unnecessary toxicity in patients with good response.
Prognostic PET-biomarkers
Timing is of crucial importance when determining the value of interim FDG-PET/CT for various reasons. First, radiotherapy-induced mucositis occurring mostly in the second half of the treatment course could complicate the interpretation of images. Secondly, evaluation should not be done too early because it takes time to develop a response. And third, the earlier response prediction can be done, the more time remains to modify the treatment strategy. There are good indications that week 2-3 of radiotherapy, corresponding to a delivered dose of about 20 Gy, seems the most favourable time-point for interim PET-evaluation[44,45,47].
In a meta-analysis, pre-treatment high metabolic tumor volume, defined as the sum of the volume of voxels in a tumor with standardized uptake value (SUV) surpassing a certain threshold value, was significantly associated with a worse loco-regional control and overall survival[46]. Several studies reported that a lower total lesion glycolysis (i.e., the product of SUVmean and metabolic tumor volume) at 3 weeks into treatment was predictive for loco-regional control and overall survival in patients with HNSCC[45,48-50]. Metabolic tumor volume and SUVmax reduction during treatment seem also prognostic for loco-regional control[45,51,52]. Hentschel et al.[47] found in a retrospective analysis that patients with a reduction of SUVmax ≥ 50% within the first 2 weeks of (C)RT, showed significantly higher 2-year overall survival rates (88% vs. 38%; P = 0.02) and 2-year loco-regional control rates (88% vs. 40%; P = 0.06) compared to patients with a SUVmax reduction < 50%. However, evidence regarding this issue remains equivocal. At present, no firm conclusions can be drawn about the optimal metabolic parameter to predict outcome early during treatment using FDG-PET, nor the corresponding threshold values.
PET-guided adaptive radiotherapy
Early response evaluation with PET imaging could open a window of opportunity for adaptive treatment strategies [Table 1][53]. First, there is the possibility to refine the target volume during the course of radiation therapy based on changes in FDG-uptake, which would allow for a reduction of the treated volume and thus facilitate healthy tissue sparing. Already in 2007, Geets et al.[54] found that, using a gradient-based algorithm on five FDG-PET scans performed before and during radiotherapy, PET-segmented target volumes could be reduced by 15%-40% compared to baseline CT-planning. Other studies have demonstrated a reduction of the mean parotid dose with CT-based re-planning during the course of radiation for a selected group of patients, which resulted in a significantly lower risk of xerostomia[53,55]. The main difficulty of anatomically adaptive radiotherapy is the fact that re-simulating, re-contouring, and re-planning of patients remains highly time-consuming[56]. Besides, there is no clear consensus about clinical or dosimetric criteria to select patients who benefit most from re-planning. However, we envisage that this process will be largely automated in the near future, based on technological advancement such as synthetic CT generation from daily cone-beam computed tomography and auto-contouring using artificial intelligence[57,58]. Though CT-based automated re-planning could theoretically be performed on a daily basis, this is not realistic for FDG-PET-guided re-planning. FDG-PET should be reserved for re-planning based on metabolic tumor response once or twice during a course of radiotherapy[44].
Second, there is the strategy of radiotherapy dose (de-)escalation during treatment. Several studies confirmed the feasibility of FDG-PET-guided “dose painting by numbers”, where voxel-wise dose escalation is related to FDG-uptake to produce a non-uniform dose distribution[59-61]. Currently two randomized controlled phase II trials are investigating whether radiotherapy dose escalation based on interim FDG-PET/CT can improve loco-regional control compared to standard radiotherapy (NCT01341535 & NCT01504815). However, in this era of concomitant chemotherapy, immunotherapy, and targeted therapy, it is debatable if radiotherapy dose escalation is a reasonable approach to improve outcome, especially since it may increase the risk of severe late toxicity such as mucosal ulcers[62]. Therefore, we believe it is important to keep the high dose volume (e.g., > 70 Gy) to a minimum. When using modern imaging, the target definition is more accurate and GTV-CTVgross margins can be tighter[63-65]. Also, the traditional dose of 70 Gy may be unnecessarily high for microscopic local tumor spread. Applying an intermediate dose of approximately 50-60 Gy to the periphery of the tumor may be adequate to eradicate small tumor extensions. This opens an opportunity for dose escalation to smaller, but highly active metabolic tumor volumes, or small tumor volumes that show residual FDG uptake towards the end of the radiation course, without increasing the risk of late squealy. In the future of HNSCC treatment, it remains highly important to explore the landscape of patient tailored radiotherapy-drug combinations and how PET-based early response assessment, next to genetic and biological tumor profiling, can steer this process[66].
Opposite to radiotherapy dose intensification, interim PET treatment evaluation enables the opportunity for dose de-escalation in patients with favourable prognosis and excellent intra-treatment tumor response in order to decrease radiotherapy related morbidity. Clinical evidence regarding interim PET-guided dose de-escalation in well-responding patients remains scarce, especially in HPV-negative HNSCC and patients treated with primary radiotherapy[67]. Recently, a non-randomized controlled trial has been initiated to evaluate the safety of radiotherapy dose de-escalation in HPV-associated oropharyngeal cancer, based on interim evaluation with FDG-PET/CT in week 2 of (C)RT (NCT04667585). Besides FDG, there are several other PET-tracers that can be useful for intra-treatment tumor response evaluation and adaptive radiotherapy, such as fluorothymidine (FLT), fluoro-ethyl-tyrosine (FET), fluoromisonidazole (FMISO) and fluoroazomycin-arabinoside (FAZA)[21,44,67-69]. Similar to FDG, week 2-3 of radiotherapy seems to be the optimal time-point for interim PET-evaluation with these tracers. Further discussion of these tracers is outside the scope of this review.
CONCLUSION
The use of FDG-PET/CT in patients with HNSCC has major implications for diagnostic staging and radiotherapy. High sensitivity of FDG-PET/CT for identification of smaller lymph node metastases not only has consequences for radiotherapy target volume definition and dose prescription, but also has prognostic implications. The use of FDG-PET/CT in radiotherapy planning opens a window of opportunity for treatment de-intensification of the neck in order to decrease toxicity without compromising oncological outcome. This concept is currently being investigated in the randomized controlled UPGRADE-RT trial. Further prospective trials investigating adaptive radiotherapy based on interim PET-evaluation are needed, especially regarding HPV-negative HNSCC and patients treated with primary radiotherapy.
DECLARATIONS
Authors’ contributionsPerformed the literature search and drafted and revised the manuscript: Cox MC, van den Bosch S
Critically reviewed and edited the manuscript: van den Bosch S, Dijkema T, Kaanders JHAM
Supervised the writing process: Kaanders JHAM
Approved the final version of the report: Cox MC, van den Bosch S, Dijkema T, Kaanders JHAM
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflict of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2021.
REFERENCES
1. Brizel DM, Albers ME, Fisher SR, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med 1998;338:1798-804.
2. Pignon JP, le Maître A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92:4-14.
3. Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003;349:2091-8.
4. Maccomb WS, Fletcher GH. Planned combination of surgery and radiation in treatment of advanced primary head and neck cancers. Am J Roentgenol Radium Ther Nucl Med 1957;77:397-414.
6. de Bondt RB, Nelemans PJ, Bakers F, et al. Morphological MRI criteria improve the detection of lymph node metastases in head and neck squamous cell carcinoma: multivariate logistic regression analysis of MRI features of cervical lymph nodes. Eur Radiol 2009;19:626-33.
7. Takes RP, Knegt P, Manni JJ, et al. Regional metastasis in head and neck squamous cell carcinoma: revised value of US with US-guided FNAB. Radiology 1996;198:819-23.
8. de Bondt RB, Nelemans PJ, Hofman PA, et al. Detection of lymph node metastases in head and neck cancer: a meta-analysis comparing US, USgFNAC, CT and MR imaging. Eur J Radiol 2007;64:266-72.
9. Zhou SM, Wong TZ, Marks LB. Using FDG-PET activity as a surrogate for tumor cell density and its effect on equivalent uniform dose calculation. Med Phys 2004;31:2577-83.
10. Kim SJ, Pak K, Kim K. Diagnostic accuracy of F-18 FDG PET or PET/CT for detection of lymph node metastasis in clinically node negative head and neck cancer patients; A systematic review and meta-analysis. Am J Otolaryngol 2019;40:297-305.
11. Sun R, Tang X, Yang Y, Zhang C. (18)FDG-PET/CT for the detection of regional nodal metastasis in patients with head and neck cancer: a meta-analysis. Oral Oncol 2015;51:314-20.
12. Yongkui L, Jian L, Wanghan, Jingui L. 18FDG-PET/CT for the detection of regional nodal metastasis in patients with primary head and neck cancer before treatment: a meta-analysis. Surg Oncol 2013;22:e11-6.
13. Lowe VJ, Duan F, Subramaniam RM, et al. Multicenter trial of [18F]fluorodeoxyglucose positron emission tomography/computed tomography staging of head and neck cancer and negative predictive value and surgical impact in the N0 neck: results from ACRIN 6685. J Clin Oncol 2019;37:1704-12.
14. Roh JL, Park JP, Kim JS, et al. 18F fluorodeoxyglucose PET/CT in head and neck squamous cell carcinoma with negative neck palpation findings: a prospective study. Radiology 2014;271:153-61.
15. Yamazaki Y, Saitoh M, Notani K, et al. Assessment of cervical lymph node metastases using FDG-PET in patients with head and neck cancer. Ann Nucl Med 2008;22:177-84.
16. Yoon DY, Hwang HS, Chang SK, et al. CT, MR, US,18F-FDG PET/CT, and their combined use for the assessment of cervical lymph node metastases in squamous cell carcinoma of the head and neck. Eur Radiol 2009;19:634-42.
17. Ong SC, Schöder H, Lee NY, et al. Clinical utility of 18F-FDG PET/CT in assessing the neck after concurrent chemoradiotherapy for Locoregional advanced head and neck cancer. J Nucl Med 2008;49:532-40.
18. Schöder H, Fury M, Lee N, Kraus D. PET monitoring of therapy response in head and neck squamous cell carcinoma. J Nucl Med 2009;50 Suppl 1:74S-88S.
19. Helsen N, Van den Wyngaert T, Carp L, Stroobants S. FDG-PET/CT for treatment response assessment in head and neck squamous cell carcinoma: a systematic review and meta-analysis of diagnostic performance. Eur J Nucl Med Mol Imaging 2018;45:1063-71.
20. Van den Wyngaert T, Helsen N, Carp L, et al. Fluorodeoxyglucose-positron emission tomography/computed tomography after concurrent chemoradiotherapy in locally advanced head-and-neck squamous cell cancer: The ECLYPS Study. J Clin Oncol 2017;35:3458-64.
21. Bussink J, van Herpen CM, Kaanders JH, Oyen WJ. PET-CT for response assessment and treatment adaptation in head and neck cancer. Lancet Oncol 2010;11:661-9.
22. Mehanna H, Wong WL, McConkey CC, et al. PET-CT surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med 2016;374:1444-54.
23. Dornfeld K, Hopkins S, Simmons J, et al. Posttreatment FDG-PET uptake in the supraglottic and glottic larynx correlates with decreased quality of life after chemoradiotherapy. Int J Radiat Oncol Biol Phys 2008;71:386-92.
24. Delouya G, Igidbashian L, Houle A, et al. 18F-FDG-PET imaging in radiotherapy tumor volume delineation in treatment of head and neck cancer. Radiother Oncol 2011;101:362-8.
25. Guido A, Fuccio L, Rombi B, et al. Combined 18F-FDG-PET/CT imaging in radiotherapy target delineation for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2009;73:759-63.
26. Koshy M, Paulino AC, Howell R, Schuster D, Halkar R, Davis LW. F-18 FDG PET-CT fusion in radiotherapy treatment planning for head and neck cancer. Head Neck 2005;27:494-502.
27. Mazzola R, Alongi P, Ricchetti F, et al. 18F-Fluorodeoxyglucose-PET/CT in locally advanced head and neck cancer can influence the stage migration and nodal radiation treatment volumes. Radiol Med 2017;122:952-9.
28. van den Bosch S, Doornaert PAH, Dijkema T, et al. 18F-FDG-PET/CT-based treatment planning for definitive (chemo)radiotherapy in patients with head and neck squamous cell carcinoma improves regional control and survival. Radiother Oncol 2020;142:107-14.
29. van den Bosch S, Vogel WV, Raaijmakers CP, et al. Implications of improved diagnostic imaging of small nodal metastases in head and neck cancer: radiotherapy target volume transformation and dose de-escalation. Radiother Oncol 2018;128:472-8.
30. den Bosch S, Dijkema T, Verhoef LC, Zwijnenburg EM, Janssens GO, Kaanders JH. Patterns of recurrence in electively irradiated lymph node regions after definitive accelerated intensity modulated radiation therapy for head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2016;94:766-74.
31. van den Bosch S, Dijkema T, Philippens MEP, et al. Tumor to cervical spinal cord standardized uptake ratio (SUR) improves the reproducibility of 18F-FDG-PET based tumor segmentation in head and neck squamous cell carcinoma in a multicenter setting. Radiother Oncol 2019;130:39-45.
32. van den Bosch S, Dijkema T, Kunze-Busch MC, et al. Uniform FDG-PET guided GRAdient Dose prEscription to reduce late Radiation Toxicity (UPGRADE-RT): study protocol for a randomized clinical trial with dose reduction to the elective neck in head and neck squamous cell carcinoma. BMC Cancer 2017;17:208.
33. Withers H, Peters LJ, Taylor JM. Dose-response relationship for radiation therapy of subclinical disease. Int J Radiat Oncol Biol Phys 1995;31:353-9.
34. Kaanders JHAM, van den Bosch S, Dijkema T, Al-Mamgani A, Raaijmakers CPJ, Vogel WV. Advances in cancer imaging require renewed radiotherapy dose and target volume concepts. Radiother Oncol 2020;148:140-2.
35. Deschuymer S, Nevens D, Duprez F, et al. Randomized clinical trial on reduction of radiotherapy dose to the elective neck in head and neck squamous cell carcinoma; update of the long-term tumor outcome. Radiother Oncol 2020;143:24-9.
36. Sher DJ, Pham NL, Shah JL, et al. Prospective phase 2 study of radiation therapy dose and volume de-escalation for elective neck treatment of oropharyngeal and laryngeal cancer. Int J Radiat Oncol Biol Phys 2021;109:932-40.
37. Deschuymer S, Nevens D, Duprez F, et al. Randomized clinical trial on reduction of radiotherapy dose to the elective neck in head and neck squamous cell carcinoma: results on the quality of life. Qual Life Res 2021;30:117-27.
38. Nevens D, Duprez F, Daisne JF, et al. Reduction of the dose of radiotherapy to the elective neck in head and neck squamous cell carcinoma; a randomized clinical trial. Effect on late toxicity and tumor control. Radiother Oncol 2017;122:171-7.
39. Maguire PD, Neal CR, Hardy SM, Schreiber AM. Single-arm phase 2 trial of elective nodal dose reduction for patients with locoregionally advanced squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2018;100:1210-6.
40. Tsai CJ, McBride SM, Riaz N, Lee NY. Reducing the radiation therapy dose prescription for elective treatment areas in human papillomavirus-associated oropharyngeal carcinoma being treated with primary chemoradiotherapy at memorial sloan kettering cancer center. Pract Radiat Oncol 2019;9:98-101.
41. Driessen DAJJ, Dijkema T, Weijs WLJ, et al. Novel diagnostic approaches for assessment of the clinically negative neck in head and neck cancer patients. Front Oncol 2020;10:637513.
43. Pollom EL, Song J, Durkee BY, et al. Prognostic value of midtreatment FDG-PET in oropharyngeal cancer. Head Neck 2016;38:1472-8.
44. Garibaldi C, Ronchi S, Cremonesi M, et al. Interim 18F-FDG PET/CT during chemoradiation therapy in the management of head and neck cancer patients: a systematic review. Int J Radiat Oncol Biol Phys 2017;98:555-73.
45. Martens RM, Noij DP, Ali M, et al. Functional imaging early during (chemo)radiotherapy for response prediction in head and neck squamous cell carcinoma; a systematic review. Oral Oncol 2019;88:75-83.
46. Bonomo P, Merlotti A, Olmetto E, et al. What is the prognostic impact of FDG PET in locally advanced head and neck squamous cell carcinoma treated with concomitant chemo-radiotherapy? Eur J Nucl Med Mol Imaging 2018;45:2122-38.
47. Hentschel M, Appold S, Schreiber A, et al. Early FDG PET at 10 or 20 Gy under chemoradiotherapy is prognostic for locoregional control and overall survival in patients with head and neck cancer. Eur J Nucl Med Mol Imaging 2011;38:1203-11.
48. Kim S, Oh S, Kim JS, et al. Prognostic value of FDG PET/CT during radiotherapy in head and neck cancer patients. Radiat Oncol J 2018;36:95-102.
49. Lin P, Min M, Lee M, et al. Nodal parameters of FDG PET/CT performed during radiotherapy for locally advanced mucosal primary head and neck squamous cell carcinoma can predict treatment outcomes: SUVmean and response rate are useful imaging biomarkers. Eur J Nucl Med Mol Imaging 2017;44:801-11.
50. Min M, Lin P, Lee MT, et al. Prognostic role of metabolic parameters of (18)F-FDG PET-CT scan performed during radiation therapy in locally advanced head and neck squamous cell carcinoma. Eur J Nucl Med Mol Imaging 2015;42:1984-94.
51. Chen SW, Hsieh TC, Yen KY, et al. Interim FDG PET/CT for predicting the outcome in patients with head and neck cancer. Laryngoscope 2014;124:2732-8.
52. Min M, Lin P, Lee M, et al. Prognostic value of 2-[(18)F] fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography scan carried out during and after radiation therapy for head and neck cancer using visual therapy response interpretation criteria. Clin Oncol (R Coll Radiol) 2016;28:393-401.
54. Geets X, Tomsej M, Lee JA, et al. Adaptive biological image-guided IMRT with anatomic and functional imaging in pharyngo-laryngeal tumors: impact on target volume delineation and dose distribution using helical tomotherapy. Radiother Oncol 2007;85:105-15.
55. Castelli J, Simon A, Louvel G, et al. Impact of head and neck cancer adaptive radiotherapy to spare the parotid glands and decrease the risk of xerostomia. Radiat Oncol 2015;10:6.
56. Vorwerk H, Zink K, Schiller R, et al. Protection of quality and innovation in radiation oncology: the prospective multicenter trial the German Society of Radiation Oncology (DEGRO-QUIRO study). Evaluation of time, attendance of medical staff, and resources during radiotherapy with IMRT. Strahlenther Onkol 2014;190:433-43.
57. Chen L, Liang X, Shen C, Jiang S, Wang J. Synthetic CT generation from CBCT images via deep learning. Med Phys 2020;47:1115-25.
58. van Dijk LV, Van den Bosch L, Aljabar P, et al. Improving automatic delineation for head and neck organs at risk by Deep Learning Contouring. Radiother Oncol 2020;142:115-23.
59. Madani I, Duprez F, Boterberg T, et al. Maximum tolerated dose in a phase I trial on adaptive dose painting by numbers for head and neck cancer. Radiother Oncol 2011;101:351-5.
60. Berwouts D, Olteanu LA, Duprez F, et al. Three-phase adaptive dose-painting-by-numbers for head-and-neck cancer: initial results of the phase I clinical trial. Radiother Oncol 2013;107:310-6.
61. Gouw ZAR, La Fontaine MD, Vogel WV, van de Kamer JB, Sonke JJ, Al-Mamgani A. Single-center prospective trial investigating the feasibility of serial FDG-PET guided adaptive radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys 2020;108:960-8.
62. Berwouts D, Madani I, Duprez F, et al. Long-term outcome of 18F-fluorodeoxyglucose-positron emission tomography-guided dose painting for head and neck cancer: Matched case-control study. Head Neck 2017;39:2264-75.
63. Fleury B, Thariat J, Barnoud R, et al. Microscopic extensions of head and neck squamous cell carcinomas: impact for clinical target volume definition. Cancer Radiother 2014;18:666-71.
64. Ligtenberg H, Jager EA, Caldas-Magalhaes J, et al. Modality-specific target definition for laryngeal and hypopharyngeal cancer on FDG-PET, CT and MRI. Radiother Oncol 2017;123:63-70.
65. Grégoire V, Evans M, Le QT, et al. Delineation of the primary tumour Clinical Target Volumes (CTV-P) in laryngeal, hypopharyngeal, oropharyngeal and oral cavity squamous cell carcinoma: AIRO, CACA, DAHANCA, EORTC, GEORCC, GORTEC, HKNPCSG, HNCIG, IAG-KHT, LPRHHT, NCIC CTG, NCRI, NRG Oncology, PHNS, SBRT, SOMERA, SRO, SSHNO, TROG consensus guidelines. Radiother Oncol 2018;126:3-24.
66. Bristow RG, Alexander B, Baumann M, et al. Combining precision radiotherapy with molecular targeting and immunomodulatory agents: a guideline by the American Society for Radiation Oncology. Lancet Oncol 2018;19:e240-51.
67. Lee N, Schoder H, Beattie B, et al. Strategy of using intratreatment hypoxia imaging to selectively and safely guide radiation dose de-escalation concurrent with chemotherapy for locoregionally advanced human papillomavirus-related oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2016;96:9-17.
68. Troost EG, Bussink J, Hoffmann AL, Boerman OC, Oyen WJ, Kaanders JH. 18F-FLT PET/CT for early response monitoring and dose escalation in oropharyngeal tumors. J Nucl Med 2010;51:866-74.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Cox MC, van den Bosch S, Dijkema T, Kaanders JHAM. 18F-FDG-PET/CT-guided radiotherapy of cervical lymph nodes in head and neck squamous cell carcinoma. J Cancer Metastasis Treat 2021;7:25. http://dx.doi.org/10.20517/2394-4722.2021.55
AMA Style
Cox MC, van den Bosch S, Dijkema T, Kaanders JHAM. 18F-FDG-PET/CT-guided radiotherapy of cervical lymph nodes in head and neck squamous cell carcinoma. Journal of Cancer Metastasis and Treatment. 2021; 7: 25. http://dx.doi.org/10.20517/2394-4722.2021.55
Chicago/Turabian Style
Cox, Maurice C., Sven van den Bosch, Tim Dijkema, Johannes H.A.M. Kaanders. 2021. "18F-FDG-PET/CT-guided radiotherapy of cervical lymph nodes in head and neck squamous cell carcinoma" Journal of Cancer Metastasis and Treatment. 7: 25. http://dx.doi.org/10.20517/2394-4722.2021.55
ACS Style
Cox, MC.; van den Bosch S.; Dijkema T.; Kaanders JHAM. 18F-FDG-PET/CT-guided radiotherapy of cervical lymph nodes in head and neck squamous cell carcinoma. J. Cancer. Metastasis. Treat. 2021, 7, 25. http://dx.doi.org/10.20517/2394-4722.2021.55
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 3 clicks
Cite This Article 3 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.