Progress in the treatment of NK-cell lymphoma/leukemia
Abstract
Natural killer (NK)/T cell lymphoma includes two major subtypes of disease, specifically extranodal NK/T cell lymphoma, nasal type (ENKL) and aggressive NK cell leukemia (ANKL). Both are strongly associated with Epstein-Barr virus and are prevalent in East Asia and Latin America. Except for that of limited-stage ENKL, the prognosis of both diseases was poor in the previous decade. The advent of non-anthracycline-based chemoradiotherapy has contributed to an improvement in ENKL prognosis, but there is still room for further treatment progress. Recently, the high efficacy of PD-1 antibody was reported in relapsed or refractory ENKL patients. This was later supported by the finding that PD-L1/PD-L2 genetic alterations are frequently observed in ENKL and ANKL patients. Due to the rarity of the disease, a standard treatment for ANKL remains to be established. Currently, allogeneic stem cell transplantation is the only curative treatment, and this is even applicable to chemo-resistant ANKL patients. In this review, we focus on recent treatment approaches for NK/T cell lymphomas including novel agents.
Keywords
INTRODUCTION
Natural killer (NK) cell neoplasms are rare hematological malignancies, with two major subtypes of extranodal NK/T cell lymphoma, nasal type (ENKL) and aggressive NK-cell leukemia (ANKL)[1]. Both ENKL and ANKL are strongly associated with Epstein-Barr virus (EBV)[1]. The geographic distribution of ENKL is distinctive, with a particularly high prevalence in East Asia and South America. ENKL corresponds to approximately 2%-11% of cases among all lymphoma subtypes in East Asian countries, whereas the prevalence is less than 1% in the United States and Europe[2-4]. Although the distribution and incidence of ANKL are unclear due to its rarity, the prevalence is approximately one-sixth that of ENKL, accounting for fewer than 1% of cases among all malignant lymphomas[5]. ENKL is distinct from other lymphomas based on its unique characteristics, comprising the dominant involvement of the nasal cavity and nasopharynx. The majority of ENKL cases are limited to a location around the nose or upper aerodigestive tract, presenting with typical symptoms of nasal obstruction, epistaxis, or rhinorrhea. In contrast, ANKL is prevalent in the peripheral blood, bone marrow, liver, and spleen. Most patients suffer general symptoms, such as fever, general malaise, and loss of appetite. Clinical courses of advanced-stage ENKL and ANKL are very aggressive, often leading to disseminated intravascular coagulation and multiorgan dysfunction, thus resulting in a poor prognosis over the past decade. Recently, with an increasing understanding of the molecular pathogenesis of NK cell neoplasms, novel treatment strategies including targeted agents are expected to improve survival. In this review, we highlight recent clinical findings for ENKL and ANKL and discuss future prospects.
CLINICAL CHARACTERISTICS OF NK/T CELL LYMPHOMA/LEUKEMIA
The annual incidence of ENKL varies by ethnicity and geographically from 0.06 in the United States to 0.25 in Hong Kong per 100,000 population[3]. ENKL predominantly occurs in middle-aged individuals with a median age of 52-58 years[5-8]. The incidence is 1.5 times higher in males. Because ENKL commonly develops in extranodal sites and approximately 10% of ENKL cases are derived from γδ or cytotoxic T cells[9], the disease is termed extranodal NK/T cell lymphoma in the World Health Organization (WHO) classification. The nasal or paranasal cavities and the upper aerodigestive tract are involved in more than 80% of ENKL cases, with approximately 70% of these being in a limited stage at diagnosis[5]. The patient’s initial complaints are generally focal symptoms such as nasal obstruction, epistaxis, and rhinorrhea. In contrast, the remaining cases develop in extra-nasal sites such as the skin, gastrointestinal tract, lung, or other uncommon organs[5,7]. Approximately 60% of cases with an extra-nasal origin are at an advanced stage at diagnosis. Because ENKL develops predominantly in extranodal sites, stage III cases are rare, occurring in fewer than 5% of all cases. Therefore, most cases in advanced stage are diagnosed as stage IV. These cases follow an aggressive clinical course with hemophagocytic syndrome and/or disseminated intravascular coagulation.
ANKL is a rare leukemic form of NK cell neoplasms, which has been reported mainly in East Asian countries such as Japan, China, or South Korea. The incidence is 2-3 times higher in males. ANKL progresses very rapidly, and its very aggressive clinical course is reflected by its name, aggressive NK-cell leukemia[10]. The median overall survival (OS) of ANKL is only a few months after diagnosis, which is the worst prognosis among all lymphoma subtypes[11]. Interestingly, however, a subset of ANKL shows a subacute clinical course. These subacute-type patients demonstrate female predominance and better prognosis with a median OS of more than six months. The median age for ANKL development is 40-42 years old, which is more than 10 years younger than that of ENKL, but it is also distributed in an elderly population, older than 70 years of age[5,12]. Although most ANKL cases develop de novo, there are some cases that originate from EBV-associated T/NK lymphoproliferative disease, including chronic active EBV infection, which generally develops in a younger population[13]. Unlike ENKL, the clinical symptoms of ANKL are non-specific, including fever, general malaise, and loss of appetite. Most patients have hepatomegaly and splenomegaly; thus, increased levels of lactate dehydrogenase and liver enzymes are commonly observed. Therefore, the accurate diagnosis of ANKL is often difficult, and a postmortem diagnosis is sometimes made after an inaccurate diagnosis of acute hepatitis. Complete blood counts show pancytopenia, and the morphology of leukemic cells varies widely, from normal large granular lymphocyte to lymphoid cells with atypical nuclei and a prominent nucleolus. Occasionally, the percentage of tumor cells in the bone marrow and peripheral blood is much lower than that in acute myeloid or lymphoblastic leukemia, with median percentages of 22% and 8%, respectively[12]. Therefore, it is important to consider ANKL as a differential diagnosis particularly in cases with fever of unknown origin, hepatosplenomegaly, or liver dysfunction.
The detection of EBV is required to make a diagnosis of ENKL based on the current WHO classification[1], whereas this is not mandated for ANKL since approximately 10%-15% of ANKL cases are negative for EBV[12,14]. The differences in clinical course and prognosis between ANKL patients with and without EBV infection are also controversial[12,14,15].
PROGNOSIS AND PROGNOSTIC MODELS
Several recent reports show that the prognosis of limited-stage ENKL patients has been improved through the introduction of several effective treatments in the past decade. However, the prognosis of advanced-stage ENKL patients is still unsatisfactory[7,8,16]. The patients with limited-stage ENKL have a significantly better prognosis than those with advanced-stage ENKL[7]. In the largest retrospective study of 358 ENKL patients, the five-year OS and progression-free survival (PFS) of limited-stage ENKL patients were 68% and 56%, respectively. In addition, the prognosis was significantly better for the limited-stage ENKL patients treated between 2010 and 2013 than for those treated between 2000 and 2009 (five-year OS of 79% and 63%, respectively)[7]. One of the reasons for this survival improvement is that the rate of limited-stage patients treated with effective chemoradiotherapies has increased[16]. More recently, the prognosis of limited-stage ENKL, when achieving event-free survival at 24 months after diagnosis, was demonstrated to be almost equivalent to that of the age- and sex-matched general population[17]. In contrast, the prognosis of advanced-stage ENKL is still poor with five-year OS and PFS values of 24% and 16%, respectively[7]. Although the introduction of L-asparaginase (L-asp)-containing chemotherapy has improved the OS, significant improvements in clinical practice have not been obtained yet[16].
The most popular prognostic model for ENKL is the prognostic index of NK lymphoma (PINK)[18]. This model was developed to predict the prognosis for treatment-naïve ENKL patients who received non-anthracycline-based chemotherapies with curative intent. Based on four prognostic factors, including age older than 60 years, stage III or IV, distant lymph-node involvement, and non-nasal type disease, patients are stratified into low risk (0), intermediate risk (1), and high risk (2-4) categories, with estimated three-year OS of 81%, 62%, and 25%, respectively. PINK-E, a prognostic model including EBV-DNA data on PINK, also links risk factors and OS. The international prognostic index and NK/T cell lymphoma prognostic index, previously developed prognostic models, were based on patient data of the anthracycline era, and these do not provide an accurate prognosis for patients receiving recent non-anthracycline-based chemotherapies[7,19].
The prognosis of ENKL differs by the site of origin, specifically nasal or extranasal. The prognosis of patients with an extranasal origin is significantly worse than that of patients with a nasal origin based on several studies[6,8]. The largest prospective cohort study of ENKL patients, from the International T cell Project, showed an OS at five years of 54% in patients with nasal origin and 34% in those with extranasal origin (log-rank P = 0.019)[8]. However, the impact of the site of disease origin, nasal or extranasal, on OS is controversial. Because the percentage of advanced stage disease is generally higher in patients with extranasal origin than in those with nasal origin, the disease stage could be a confounding factor when evaluating the prognosis by the site of disease origin. In fact, another study reported that prognosis does not significantly differ between the two groups in subgroup analyses by disease stage[20].
There are no prognostic models for ANKL. In general, the prognosis of this disease is extremely poor, with a median OS of 2-3 months[5,11,12], except for that of the subacute-type ANKL[21]. Allogeneic stem cell transplantation is the only curative treatment and could be the most important factor for survival. Patients with subacute ANKL have symptoms of infectious mononucleosis for more than 90 days prior to the fulminant onset, and the prognosis is significantly better than that of typical ANKL, with a median OS of 214 days[21]. ANKL originating from EBV-associated NK/T cell lymphoproliferative diseases such as chronic active EBV infection has been reported[13], but its clinical course and prognosis are not well known.
TREATMENT OF NK-CELL LYMPHOMA
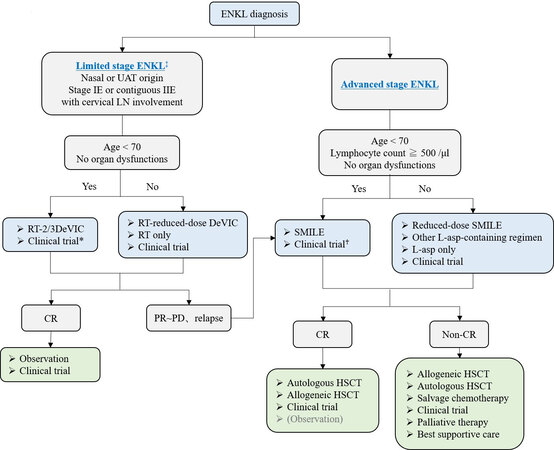
The recommended algorithm of treatments for ENKL patients is shown in Figure 1. The scheme is divided based on the extent of disease (limited vs. advanced). The history of treatment development is further shown in Table 1.
Figure 1. Treatment flowchart of ENKL patients. *CCRT-VIPD and CCRT-VIDL regimens are included. ♰AspaMetDex regimen is included only in relapsed or refractory ENKL patients. ‡The definition of limited stage in this flowchart is a little different from that in the conventional Lugano/Ann Arbor classification. UAT: Upper aerodigestive tract; LN: lymph node; RT: radiotherapy; DeVIC: dexamethasone, etoposide, ifosfamide, carboplatin; CR: complete response; PR: partial response; PD: progressive disease; SMILE: steroid, methotrexate, ifosfamide, L-asparaginase, etoposide; L-asp: L-asparaginase; HSCT: hematopoietic stem cell transplantation.
The history of treatment development for limited and advanced stage ENKL
| Published year | Treatment | Patients | n | Trial phase | ORR (%) | CR (%) | OS | PFS | Ref. | ||
| 1998 | RT | TN-stage I/II NL+ | 90 | Retrospective | NA | 74 | 48%-59% (2-year) | 41%-48% (2-year) | [24] | ||
| Limited stage | Anthracycline-era | 2000 | RT | TN-stage I/II | 92 | Retrospective | 84 | 66 | 40% (5-year) | 38% (5-year) | [25] |
| 2001 | • Frontline RT ± CT | TN-stage I/II | 8 | Retrospective | 100 | 100 | Median NR | NA | [32] | ||
| • Frontline CT (CHOP-like) ± RT | TN-stage I/II | 12 | Retrospective | 67 | 25 | Median 35 month | NA | [32] | |||
| 2001 | CHOP followed by RT 45Gy | TN-stage I/II | 17 | Retrospective | NA | 58 | 59% (3-year) | NA | [33] | ||
| 2004 | CT-> ± RT | TN-stage I/II | 40 | Retrospective | NA | 72 | 29% (5-year) | 28% (5-year) | [34] | ||
| 2004 | RT | TN-stage I/II | 102 | Retrospective | 85 | 72 | 42% (5-year) | 53% (5-year) | [26] | ||
| 2006 | RT and/or CT | TN-stage IE/IIE | 105 | retrospective | 90 | 87 | 71% (5-year) | 59% (5-year) | [22] | ||
| → Frontline CT (CHOP-like) | 40 | Retrospective | 60* | 20* | NA | NA | [22] | ||||
| 2007 | CT (CHOP) -> RT | TN-stage IE/IIE | 53 | Retrospective | NA | 49 | 76% (2-year) | 62% (2-year) | [34] | ||
| Non-anthracycline-era | 2009 | CCRT (RT-2/3DeVIC) | TN-stage IE/IIE | 33 | phase II | 81 | 77 | 70% (5-year) | 63% (5-year) | [36] | |
| 2009 | CCRT (RT-VIPD) | TN-stage IE/IIE | 30 | phase II | 83 | 80 | 86% (3-year) | 85% (3-year) | [37] | ||
| 2014 | CCRT (RT-VIDL) | TN-stage IE/IIE | 30 | phase II | 90 | 87 | 73% (5-year) | 60% (5-year) | [38] | ||
| Advanced stage | Anthracycline-era | 1995 | CHOP-like | TN-stage III/IV NL+ | 33 | Retrospective | NA | 30 | 8% (5-year) | NA | [44] |
| 1998 | CHOP-like | TN-stage III/IV | 9 | Retrospective | NA | NA | 12% (5-year) | NA | [24] | ||
| 2003 | L-asp, Dex, VCR | CHOP-like Refractory | 18 | Retrospective | 83 | 56 | 56% (5-year) | NA | [48] | ||
| 2010 | CHOP-like | TN-stage IV | 47 | Retrospective | 36 | 10 | 10% (5-year) | NA | [5] | ||
| Non-anthracycline-era | 2011 | SMILE | R/R or stage IV | 38 | Phase II | 79 | 45 | 50% (5-year) | NA | [42] | |
| 2011 | AspaMetDex | R/R | 19 | Phase II | 78 | 61 | Median 12 month | median 12 month | [53] | ||
| 2016 | P-GEMOX | R/R or TN-stage III/IV | 35 | Retrospective | 80 | 51 | 65% (3-year) | 39% (3-year) | [51] | ||
| 2016 | Modified SMILE | TN-stage III/IV | 9 | Retrospective | 22 | NA | NA | NA | [50] |
Limited-stage ENKL
For limited-stage ENKL, concurrent chemoradiotherapy (CCRT) is recommended as a first-line therapy. Radiotherapy (RT) has been used as an effective treatment for limited-stage ENKL since the 1980s[22]. Although RT-containing treatment provides significantly better outcomes for limited-stage ENKL[23], RT alone frequently induces local or systemic treatment failure in more than 50% of patients[24-26]. Several studies have shown that the recommended dose of RT that is necessary for local control of limited-stage ENKL is 50 Gy or more[23,27,28]. A combination of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and RT is an established treatment for localized non-Hodgkin lymphoma, and it is also used to treat ENKL patients[29]. However, because normal and abnormal NK cells express multidrug resistant (MDR)-associated P-glycoprotein[30,31], anthracycline-containing regimens such as CHOP are not satisfactory for treating ENKL patients, with a five-year OS of less than 50%, even combined with RT[6,24,32,33]. In addition, chemotherapy followed by RT was shown to yield a worse response rate than RT followed by chemotherapy for limited-stage ENKL in a few studies[22,34,35]. Based on these experiences, several CCRT regimens have been developed as novel anti-MDR treatments for limited-stage ENKL patients since the early 2000s[36-38]. These regimens consist of non-MDR-associated anti-cancer agents, such as platinum derivatives, and ifosfamide (IFM), L-asp which has in vitro sensitivity against an NK cell tumor cell line[39], or etoposide (ETP) which is known to be effective against EBV-associated hemophagocytic syndrome[40,41]. One of the most popular CCRT regimens is RT-2/3DeVIC (dexamethasone, ETP, IFM, and carboplatin)[36], which includes concurrent RT of 50-50.4 Gy in 25-28 fractions with three cycles of a two-third dose of DeVIC. The efficacy of RT-2/3DeVIC therapy for newly diagnosed stage IE or contiguous IIE ENKL patients is better with a two-year OS of 78% and an overall response rate (ORR) of 81%, relative to the historical data. The adverse events (AEs) of RT-2/3DeVIC are acceptable. There have been no treatment-related deaths and only neutropenia was reported as a Grade 4 AE, developing in more than 20% of the patients. Other CCRT regimens used in Korea, such as CCRT-VIPD [ETP, IFM, cisplatin (CDDP), and dexamethasone (DEX)] and CCRT-VIDL (ETP, IFM, DEX, and L-asp)[37,38], have common characteristics such as RT with a median of 40 Gy conducted with weekly CDDP monotherapy, and then combination chemotherapy is conducted after RT completion. VIPD is given every three weeks for three cycles after 3-5 weeks from the time of CCRT completion[37]. VIDL is similar to VIPD, but L-asp is used instead of CDDP every other day from Day 8 to Day 20, as in the SMILE regimen[38,42]. VIDL is given every three weeks for two cycles after 3-4 weeks from the time of CCRT completion[38]. Compared to the RT-2/3DeVIC regimen, these regimens include a lower dose of RT and take a longer time to complete the treatment, but both results are good and comparable to those of RT-2/3DeVIC. VIDL shows less hematologic toxicity than VIPD, but there are no trials comparing these regimens. RT-2/3DeVIC is the most commonly used regimen in Japan. Recently, its durable response was reported with a five-year OS of 70%[7]. Although there are several sequential chemoradiotherapies, no significant difference in OS has been observed compared to that with CCRT[43], and no studies comparing an efficacy of each regimen have been reported.
Advanced-stage ENKL
For advanced stage ENKL, SMILE [steroid (DEX), methotrexate (MTX), IFM, L-asp, and ETP] chemotherapy is the most recommended regimen as a first-line therapy. Compared to that with peripheral T-cell lymphoma, because of the MDR-associated P-glycoprotein described previously herein, CHOP is much less effective for advanced-stage ENKL patients, with a five-year OS of only 8%-12%[5,24,44]. The durable efficacy of L-asp monotherapy for CHOP-resistant ENKL patients was reported in several cases[45,46], and L-asp-containing combination chemotherapy has been developed[47-49]. The SMILE regimen, which is a non-anthracycline-containing and non-MDR-associated agent-containing combination chemotherapy, is the standard treatment for treatment-naïve advanced-stage, relapsed, or refractory ENKL[42]. In a phase 2 study, SMILE dramatically improved survival outcomes, with a one-year OS and ORR of 55% and 79%, respectively. Peg-asparaginase-containing regimens, including modified SMILE or P-GEMOX[50,51], were also reported as effective treatments for advanced-stage, relapsed, or refractory ENKL, although the efficacy of these regimens was only evaluated retrospectively. The AspaMetDex (L-asp, MTX, and DEX) regimen is also listed as a suggested treatment for ENKL in the NCCN Guidelines[52]. The phase 2 study of AspaMetDex showed that the ORR was 78% and the one-year OS was 47% for relapsed or refractory ENKL patients[53]. However, the results of this regimen for treatment-naïve ENKL were disappointing with a high relapse rate because an antibody against L-asp, inhibiting its action, was observed in more than 70% of patients after the first cycle and eventually observed in all patients[54]. Therefore, AspaMetDex should only be used in a relapsed or refractory setting of the disease. Only a prospective clinical trial comparing the efficacy of DDGP (DEX, CDDP, gemcitabine, and peg-asparaginase) and SMILE has been reported from China, but the results cannot be evaluated owing to the poor study design[55]. The authors included a larger proportion of stage III ENKL patients than that found in the historical data (usually less than 20% of advanced-stage ENKL), and the methods and patient selection criteria described were different from the registered data on ClinicalTrials.gov (NCT01501149). Therefore, we conclude that the recommended standard treatment for advanced-stage ENKL is still the SMILE regimen[42].
Hematopoietic stem cell transplantation for ENKL
Hematopoietic stem cell transplantation (HSCT) is recommended for relapsed or refractory limited-stage ENKL, in which a complete response (CR) is not achieved after the first-line treatment, as well as for advanced-stage ENKL. The advantage of upfront HSCT has not been established in limited-stage ENKL patients or ENKL patients with favorable risk factors who achieved first CR after induction therapy[56]. In contrast, the results of a SMILE phase 2 study suggest that the OS of patients with advanced-stage, relapsed, or refractory ENKL is better in the HSCT group than in the non-HSCT group[42]. Therefore, HSCT remains the mainstay for advanced-stage ENKL in the first CR or partial response (PR). Although autologous HSCT and allogeneic HSCT have not been compared, generally, autologous HSCT is recommended for relapsed limited-stage ENKL patients with chemo-sensitivity or advanced-stage ENKL patients who achieve first CR or PR after L-asp-containing chemotherapy, and allogeneic HSCT is recommended for the remaining patients[57]. Recently, the efficacy of four cycles of VIDL followed by up-front autologous HSCT in newly diagnosed advanced-stage ENKL patients was reported in Korea[58]. Seventeen of 24 patients prospectively included in this phase 2 study, who achieved CR or PR after four cycles of VIDL, finally proceeded with upfront autologous HSCT. The median duration of the response was 15.2 months. However, nine patients (53%) relapsed after HSCT and four (24%) of the nine relapsed in the central nervous system (CNS). Therefore, we have to pay attention to CNS relapse in ENKL patients who received HSCT. Further, these results suggest that advanced-stage ENKL patients, especially those with risk factors for CNS involvement, should be treated with intermediate-dose MTX-containing chemotherapy, including SMILE, and upfront HSCT[59]. The durable efficacy of allogeneic HSCT for ENKL was reported from the Center for International Blood and Marrow Transplant Research (CIBMTR) with a three-year OS of 34% and no relapse after two years from HSCT treatment[60].
ANKL
A standard treatment for ANKL has not been established, and there are no prospective clinical trials for ANKL patients due to its rarity. The anthracycline-containing regimen is not effective for ANKL, with a median OS of 2-3 months[5]. SMILE therapy is recommended as a first-line treatment for ANKL, as for advanced-stage ENKL, although the reported evidence is limited. In a small-scale retrospective study, the ORR of SMILE therapy in 13 patients with ANKL was 38%[61]. Since ANKL progresses rapidly, most patients have B symptoms, liver dysfunction, and a poor general condition at diagnosis[5,12]. The dose of anti-cancer agents in SMILE can be reduced for such comorbid patients. Another alternative is an L-asp monotherapy followed by SMILE therapy after an improvement in organ function or the general condition. One case report showed the successful treatment with L-asp monotherapy as a first-line therapy for ANKL with severe liver dysfunction at diagnosis[62]. In that case, SMILE was supplemented after the improvement of liver function with L-asp monotherapy.
Hematopoietic stem cell transplantation for ANKL
Most ANKL patients who do not receive allogeneic HSCT, even if they achieve CR after induction chemotherapy, progress to relapse disease and eventually die[5,11,12]. Therefore, allogeneic HSCT is recommended for all ANKL patients who achieve a response and are eligible for this procedure. The effectiveness of allogeneic HSCT for ANKL was first reported in 1996[63]. Small-scale retrospective studies showing durable response and long-term survival for ANKL patients who received allogeneic HSCT have been reported since then[21,64,65]. A retrospective study of 21 ANKL patients who underwent allogeneic HSCT reported from CIBMTR showed that the two-year OS was 24% and two-year relapse rate was 59%. In this study, approximately 30% of patients who achieved CR at the time of HSCT had long-term survival, whereas all who had active disease at the time of HSCT eventually died within one year due to relapse[66]. Another retrospective study reported in Korea showed that up-front allogeneic HSCT improves survival outcomes for ANKL patients[67]. In contrast, in a small-scale retrospective study in Japan, of seven patients who underwent allogeneic HSCT and had active disease at the time of HSCT, four achieved CR after HSCT and two of these four patients remained disease-free for more than two years[12]. These results suggest that allogeneic HSCT can provide long-term survival, even in patients with active disease at the time of HSCT. Recently, the impact of allogeneic HSCT for 59 ANKL patients on survival outcomes was reported in Japan[68]. The median OS and PFS were 3.9 and 2.6 months, respectively, which are consistent with the previous data from CIBMTR. The prognosis of patients who relapsed within one year after HSCT was poor, with a median OS of only 1.4 months. In contrast, if patients survived more than one year without relapse, the subsequent five-year OS from one year after allogeneic HSCT was good (85.2%). The prognosis of patients who achieved CR or PR at the time of HSCT was significantly better than that of patients without a response, which was also comparable to a previous finding (five-year OS, 40.6% vs. 16.1%, P = 0.046). Interestingly, 15 of 24 patients with primary induction failure at the time of HSCT achieved CR after allogeneic HSCT, and the prognosis of the 15 patients (five-year OS, 32.0%) was almost comparable with that of patients with CR or PR at HSCT (P = 0.95). Therefore, allogeneic HSCT should be considered for all patients with ANKL to improve survival, even for those without response at HSCT. In addition, we should try to develop novel treatments that could provide higher responses for ANKL patients, because the efficacy and response rate of the current treatments are still not satisfactory.
NOVEL AGENTS AND THE POSSIBILITY OF CHEMO-FREE TREATMENTS
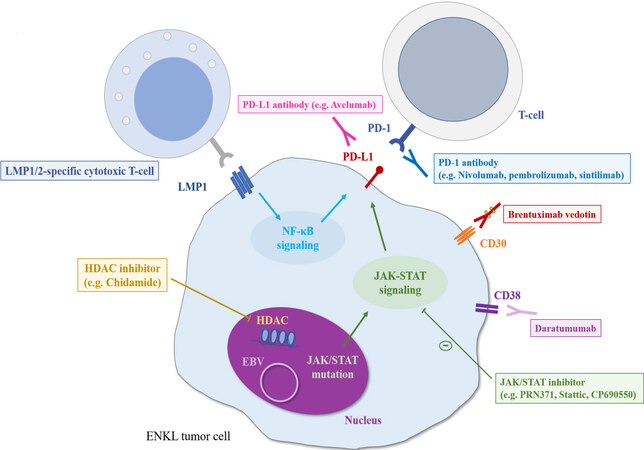
The prognosis of patients with ENKL or ANKL refractory to L-asp-based regimens is extremely poor. A scheme of the associated molecular pathway and the results of clinical trials of promising novel agents for relapsed or refractory ENKL are shown in Figure 2 and Table 2, respectively. A recent study showed that, in patients with EBV-associated lymphoma, including ENKL and ANKL, PD-L1 is frequently expressed in tumor cells owing to PD-L1/PD-L2 genetic alterations mainly in the 3’-untranslated regions (UTR)[69,70]. The PD-1/PD-L1 antibody, an immune checkpoint inhibitor, is the most promising treatment for these patients. An excellent response to pembrolizumab at 100 mg (2 mg/kg) once every three weeks in seven patients with ENKL who relapsed or were refractory to L-asp-containing regimens was reported with the ORR of 100% and CR rate of 71%, although this study had a patient selection bias[71]. Similarly, a retrospective analysis of 19 ENKL patients who were treated with pembrolizumab at 3 mg/kg once every three weeks showed that the ORR and CR rate were 47% and 37%, respectively[72]. Next-generation sequencing analysis of the samples of the 19 patients before pembrolizumab initiation in this study revealed that the gene mutation occurred most frequently in the 3’-UTR of PD-L1 (21%), which was the only therapeutic biomarker significantly associated with a good response to pembrolizumab. All ENKL patients (n = 4) with a PD-L1 mutation achieved CR with pembrolizumab and sustained the response for more than 30 months. In contrast, PD-L1 expression, evaluated by immunohistochemistry, was not significantly associated with the response to pembrolizumab[72], which was consistent with the results of another study of seven ENKL patients treated with pembrolizumab at 100 mg[73]. In this study, the ORR and CR rate were 57% and 29%, respectively[73]. Regarding other immune checkpoint inhibitors, the efficacy of low-dose nivolumab, given at 40 mg every two weeks, was also reported in a small case series[74]. In addition, sintilimab, a novel PD-1 antibody, resulted in a durable response in relapsed or refractory ENKL patients with two-year OS of 78.6% and ORR of 67.9%, as well as a manageable safety profile[75]. In contrast, a slightly lower response rate to avelumab, a PD-L1 antibody, in 21 relapsed or refractory ENKL patients was reported with the ORR of only 38%[76]. In addition, a portion of those without a response rapidly progressed. Further assessments of factors affecting the response to PD-1/PD-L1 antibodies are needed to identify the appropriate population for those treatments. More recently, the combination of several chemotherapies with immune checkpoint inhibitors has also been evaluated and has demonstrated promising results[77-79]. In particular, a phase Ib/II trial of the combination of sintilimab, a PD-1 antibody, and chidamide, a histone deacetylase (HDAC) inhibitor, in 36 relapsed or refractory ENKL patients showed superior ORR and CR rates of 58% and 44%, respectively[77]. Further analyses are warranted to identify the best combination therapy for ENKL patients.
Figure 2. The scheme of molecular pathways and promising treatments for ENKL patients. LMP: Latent membrane protein; EBV: Epstein-Barr virus; HDAC: histone deacetylase.
Clinical trials of single novel agent for relapsed or refractory ENKL patients
| Agent | Target | Trial phase | n | ORR | CR rate | Ref. |
| Pembrolizumab | PD-1 | Retrospective | 7 | 100 | 71 | [71] |
| Pembrolizumab | PD-1 | Retrospective | 19 | 47 | 37 | [72] |
| Pembrolizumab | PD-1 | Retrospective | 7 | 57 | 29 | [73] |
| Nivolumab | PD-1 | Retrospective | 3 | 67 | 67 | [74] |
| Sintilimab | PD-1 | Phase II | 28 | 68 | 14 | [75] |
| Avelumab | PD-L1 | Phase II | 21 | 38 | 24 | [76] |
| Brentuximab vedotin | CD30 | Phase II | 7 | 29 | 15 | [82] |
| Daratumumab | CD38 | Phase II | 32 | 25 | 0 | [83] |
| Chidamide | HDAC | Phase II | 79 (16) | 19 | 6 | [85] |
| LMP1/2-specific CTL | LMP1/2 | Phase II | 6 | 67 | 67 | [87] |
Several novel targeted agents other than PD-1/PD-L1 antibodies have also been evaluated for their efficacy against ENKL. The clinical efficacy of an anti-CD30 antibody-drug conjugate (brentuximab vedotin) has been reported in two CD30-positive ENKL cases[80,81]. A phase II study for relapsed or refractory CD30-positive non-Hodgkin lymphoma, including seven ENKL patients, showed an ORR of 29% and CR rate of 15%[82]. An anti-CD38 antibody (daratumumab) was also reported to have durable efficacy in a CD38-positive ENKL case[83]. A phase II study of daratumumab monotherapy conducted for 32 relapsed or refractory ENKL patients yielded the ORR of 25% and CR rate of 0% with a median PFS of 55 days[84]. Further, the HDAC inhibitor chidamide, which was developed as an oral therapeutic in China, was evaluated in a multicenter, pivotal phase II study of 79 patients with relapsed or refractory T/NK-cell lymphoma, including ENKL patients (20%). The ORR and CR rate of ENKL patients treated with chidamide were 19% and 6%, respectively[85]. In general, ENKL is strongly associated with type II EBV latency, in which latent membrane protein 1 (LMP1) and latent membrane protein 2 (LMP2) are expressed[86]. The durable efficacy of autologous LMP1/2-specific cytotoxic T cells (CTLs) has been demonstrated for relapsed or refractory ENKL patients[87]. LMP1/2-specific CTLs were also used as a consolidative therapy for high-risk ENKL patients with a complete response after induction therapy or stem cell transplantation[87,88]. In addition, based on the findings that activation of the JAK-STAT pathway, particularly associated with STAT3 and STAT5B mutations, is observed in ENKL tumor cells[89-91], the efficacy of a JAK3-selective inhibitor (PRN371)[92,93] and a STAT3 inhibitor (Stattic)[94] was reported in JAK3-mutated cell lines and a xenograft model and STAT3-mutated cell lines, respectively. Moreover, a pan-JAK inhibitor (CP-690550) reduced cell viability with increased apoptosis in both JAK3-mutated and wild-type ENKL cell lines[93]. The clinical efficacies of these agents have not been well studied, and further evaluations are needed.
There are no studies evaluating the efficacy of novel targeted agents for ANKL patients. High sensitivities to a BCL2 inhibitor and a JAK2 inhibitor in vitro were reported[95]. As described previously, because the expression of PD-L1 is high in ANKL, PD-1/PD-L1 antibodies are also promising therapeutic agents for ANKL patients with failed L-asp-containing regimen treatment, and further analysis is warranted.
CONCLUSION
The prognosis of patients with disseminated NK/T cell lymphoma is markedly improving. Recent advances contribute to the improvement, but there remains room for further remedy, particularly for patients with relapsed or refractory NK/T cell lymphoma. Developing novel agents is thus warranted.
DECLARATIONS
Authors’ contributionsWrote the manuscript: Fujimoto A
Revised the manuscript: Suzuki R
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of InterestFujimoto A declared that there are no conflicts of interest. Suzuki R received honoraria from Bristol-Myers Squibb, Novartis, Kyowa Hakko Kirin, Chugai Pharmaceuticals, Takeda, Meiji Seika Pharma, MSD, Otsuka, Celgene, Eisai Pharmaceuticals, Abbvie Inc., and Janssen outside the submitted work.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2021.
REFERENCES
1. Campo E, Harris NL, Jaffe ES, et al. . WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Geneva: WHO Press; 2008.
2. Lymphoma Study Group of Japanese Pathologists. The world health organization classification of malignant lymphomas in Japan: incidence of recently recognized entities. Pathol Int 2000;50:696-702.
3. Bassig BA, Au WY, Mang O, et al. Subtype-specific incidence rates of lymphoid malignancies in Hong Kong compared to the United States, 2001-2010. Cancer Epidemiol 2016;42:15-23.
4. Sun J, Yang Q, Lu Z, et al. Distribution of lymphoid neoplasms in China: analysis of 4,638 cases according to the World Health Organization classification. Am J Clin Pathol 2012;138:429-34.
5. Suzuki R, Suzumiya J, Yamaguchi M, et al. NK-cell Tumor Study Group. Prognostic factors for mature natural killer (NK) cell neoplasms: aggressive NK cell leukemia and extranodal NK cell lymphoma, nasal type. Ann Oncol 2010;21:1032-40.
6. Au WY, Weisenburger DD, Intragumtornchai T, et al. International Peripheral T-Cell Lymphoma Project. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood 2009;113:3931-7.
7. Yamaguchi M, Suzuki R, Oguchi M, et al. Treatments and outcomes of patients with extranodal natural killer/T-cell lymphoma diagnosed between 2000 and 2013: a cooperative study in Japan. J Clin Oncol 2017;35:32-9.
8. Fox CP, Civallero M, Ko Y, et al. Survival outcomes of patients with extranodal natural-killer T-cell lymphoma: a prospective cohort study from the international T-cell Project. Lancet Haematol 2020;7:e284-94.
9. Pongpruttipan T, Sukpanichnant S, Assanasen T, et al. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and αβ, γδ, and αβ/γδ T-cell origin: a comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol 2012;36:481-99.
10. Fernandez LA, Pope B, Lee C, Zayed E. Aggressive natural killer cell leukemia in an adult with establishment of an NK cell line. Blood 1986;67:925-30.
11. Suzuki R, Suzumiya J, Nakamura S, et al. NK-cell Tumor Study Group. Aggressive natural killer-cell leukemia revisited: large granular lymphocyte leukemia of cytotoxic NK cells. Leukemia 2004;18:763-70.
12. Ishida F, Ko YH, Kim WS, et al. Aggressive natural killer cell leukemia: therapeutic potential of L-asparaginase and allogeneic hematopoietic stem cell transplantation. Cancer Sci 2012;103:1079-83.
13. Kimura H, Ito Y, Kawabe S, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood 2012;119:673-86.
14. Ko YH, Park S, Kim K, Kim SJ, Kim WS. Aggressive natural killer cell leukemia: is Epstein-Barr virus negativity an indicator of a favorable prognosis? Acta Haematol 2008;120:199-206.
15. Nicolae A, Ganapathi KA, Pham TH, et al. EBV-negative aggressive NK-cell leukemia/lymphoma: clinical, pathologic, and genetic features. Am J Surg Pathol 2017;41:67-74.
17. Yang Y, Wang Y, Liu X, et al. Progression-free survival at 24 months and subsequent survival of patients with extranodal NK/T-cell lymphoma: a China Lymphoma Collaborative Group (CLCG) study. Leukemia 2021;35:1671-82.
18. Kim SJ, Yoon DH, Jaccard A, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol 2016;17:389-400.
19. Lee J, Suh C, Park YH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol 2006;24:612-8.
20. Suzuki R, Suzumiya J, Oshimi K. Differences between nasal and extranasal NK/T-cell lymphoma. Blood 2009;113:6260-1; author reply 6261-2.
21. Tang YT, Wang D, Luo H, et al. Aggressive NK-cell leukemia: clinical subtypes, molecular features, and treatment outcomes. Blood Cancer J 2017;7:660.
22. Li YX, Yao B, Jin J, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol 2006;24:181-9.
23. Vargo JA, Patel A, Glaser SM, et al. The impact of the omission or inadequate dosing of radiotherapy in extranodal natural killer T-cell lymphoma, nasal type, in the United States. Cancer 2017;123:3176-85.
24. Cheung MM, Chan JK, Lau WH, et al. Primary non-Hodgkin's lymphoma of the nose and nasopharynx: clinical features, tumor immunophenotype, and treatment outcome in 113 patients. J Clin Oncol 1998;16:70-7.
25. Kim GE, Cho JH, Yang WI, et al. Angiocentric lymphoma of the head and neck: patterns of systemic failure after radiation treatment. J Clin Oncol 2000;18:54-63.
26. Koom WS, Chung EJ, Yang WI, et al. Angiocentric T-cell and NK/T-cell lymphomas: radiotherapeutic viewpoints. Int J Radiat Oncol Biol Phys 2004;59:1127-37.
27. Yang CW, Wang CW, Hong RL, et al. Treatment outcomes of and prognostic factors for definitive radiotherapy with and without chemotherapy for Stage I/II nasal extranodal NK/T-cell lymphoma. J Radiat Res 2017;58:114-22.
28. Yang Y, Zhu Y, Cao JZ, et al. Risk-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma: analysis from a multicenter study. Blood 2015;126:1424-32; quiz 1517.
29. Miller TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin's lymphoma. N Engl J Med 1998;339:21-6.
30. Egashira M, Kawamata N, Sugimoto K, et al. P-glycoprotein expression on normal and abnormally expanded natural killer cells and inhibition of P-glycoprotein function by cyclosporin A and its analogue, PSC833. Blood 1999;93:599-606.
31. Yamaguchi M, Kita K, Miwa H, et al. Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer 1995;76:2351-6.
32. Ribrag V, Ell Hajj M, Janot F, et al. Early locoregional high-dose radiotherapy is associated with long-term disease control in localized primary angiocentric lymphoma of the nose and nasopharynx. Leukemia 2001;15:1123-6.
33. Ribrag V, Ell Hajj M, Janot F, et al. Early locoregional high-dose radiotherapy is associated with long-term disease control in localized primary angiocentric lymphoma of the nose and nasopharynx. Leukemia 2001;15:1123-6.
34. You JY, Chi KH, Yang MH, et al. Radiation therapy versus chemotherapy as initial treatment for localized nasal natural killer (NK)/T-cell lymphoma: a single institute survey in Taiwan. Ann Oncol 2004;15:618-25.
35. Wang B, Lu JJ, Ma X, et al. Combined chemotherapy and external beam radiation for stage IE and IIE natural killer T-cell lymphoma of nasal cavity. Leuk Lymphoma 2007;48:396-402.
36. Yamaguchi M, Tobinai K, Oguchi M, et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol 2009;27:5594-600.
37. Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell Lymphoma: consortium for Improving Survival of Lymphoma study. J Clin Oncol 2009;27:6027-32.
38. Kim SJ, Yang DH, Kim JS, et al. Concurrent chemoradiotherapy followed by L-asparaginase-containing chemotherapy, VIDL, for localized nasal extranodal NK/T cell lymphoma: CISL08-01 phase II study. Ann Hematol 2014;93:1895-901.
39. Ando M, Sugimoto K, Kitoh T, et al. Selective apoptosis of natural killer-cell tumours by l-asparaginase. Br J Haematol 2005;130:860-8.
40. Chen JS, Lin KH, Lin DT, Chen RL, Jou ST, Su IJ. Longitudinal observation and outcome of nonfamilial childhood haemophagocytic syndrome receiving etoposide-containing regimens. Br J Haematol 1998;103:756-62.
41. Uno M, Tsuchiyama J, Moriwaki A, et al. In vitro induction of apoptosis for nasal angiocentric natural killer cell lymphoma-derived cell line, NK-YS, by etoposide and cyclosporine A. Br J Haematol 2001;113:1009-14.
42. Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol 2011;29:4410-6.
43. Kwong YL, Kim SJ, Tse E, et al. Sequential chemotherapy/radiotherapy was comparable with concurrent chemoradiotherapy for stage I/II NK/T-cell lymphoma. Ann Oncol 2018;29:256-63.
44. Liang R, Todd D, Chan TK, et al. Treatment outcome and prognostic factors for primary nasal lymphoma. J Clin Oncol 1995;13:666-70.
45. Nagafuji K, Fujisaki T, Arima F, Ohshima K. L-asparaginase induced durable remission of relapsed nasal NK/T-cell lymphoma after autologous peripheral blood stem cell transplantation. Int J Hematol 2001;74:447-50.
46. Matsumoto Y, Nomura K, Kanda-Akano Y, et al. Successful treatment with Erwinia L-asparaginase for recurrent natural killer/T cell lymphoma. Leuk Lymphoma 2003;44:879-82.
47. Obama K, Tara M, Niina K. L-asparaginase-based induction therapy for advanced extranodal NK/T-cell lymphoma. Int J Hematol 2003;78:248-50.
48. Yong W, Zheng W, Zhang Y, et al. L-asparaginase-based regimen in the treatment of refractory midline nasal/nasal-type T/NK-cell lymphoma. Int J Hematol 2003;78:163-7.
49. Jaccard A, Petit B, Girault S, et al. L-asparaginase-based treatment of 15 western patients with extranodal NK/T-cell lymphoma and leukemia and a review of the literature. Ann Oncol 2009;20:110-6.
50. Qi S, Yahalom J, Hsu M, et al. Encouraging experience in the treatment of nasal type extra-nodal NK/T-cell lymphoma in a non-Asian population. Leuk Lymphoma 2016;57:2575-83.
51. Wang JH, Wang L, Liu CC, et al. Efficacy of combined gemcitabine, oxaliplatin and pegaspargase (P-gemox regimen) in patients with newly diagnosed advanced-stage or relapsed/refractory extranodal NK/T-cell lymphoma. Oncotarget 2016;7:29092-101.
52. Horwitz SM, Ansell SM, Ai WZ, et al. NCCN Guidelines Insights: T-Cell Lymphomas, Version 2.2018. J Natl Compr Canc Netw 2018;16:123-35.
53. Jaccard A, Gachard N, Marin B, et al. GELA and GOELAMS Intergroup. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood 2011;117:1834-9.
54. Jaccard A, Suarez F, Delmer A, et al. A prospective phase II trial of an L-asparaginase containing regimen in extra nodal NK/T-cell lymphoma. Hematol Oncol 2013;31:129.
55. Li X, Cui Y, Sun Z, et al. DDGP versus SMILE in newly diagnosed advanced natural killer/T-cell lymphoma: a randomized controlled, multicenter, open-label study in China. Clin Cancer Res 2016;22:5223-8.
56. Yhim HY, Kim JS, Mun YC, et al. Consortium for Improving Survival of Lymphoma Study. Clinical outcomes and prognostic factors of up-front autologous stem cell transplantation in patients with extranodal natural killer/T cell lymphoma. Biol Blood Marrow Transplant 2015;21:1597-604.
57. Kharfan-Dabaja MA, Kumar A, Ayala E, et al. Clinical Practice recommendations on indication and timing of hematopoietic cell transplantation in mature T cell and NK/T cell lymphomas: an International Collaborative Effort on Behalf of The Guidelines Committee of The American Society for blood and marrow transplantation. Biol Blood Marrow Transplant 2017;23:1826-38.
58. Song GY, Yoon DH, Suh C, et al. Open-label, single arm, multicenter phase II study of VIDL induction chemotherapy followed by upfront autologous stem cell transplantation in patients with advanced stage extranodal NK/T-cell lymphoma. Bone Marrow Transplant 2021;56:1205-8.
59. Kim H, Jeong H, Yamaguchi M, et al. Prediction and prevention of central nervous system relapse in patients with extranodal natural killer/T-cell lymphoma. Blood 2020;136:2548-56.
60. Kanate AS, DiGilio A, Ahn KW, et al. Allogeneic haematopoietic cell transplantation for extranodal natural killer/T-cell lymphoma, nasal type: a CIBMTR analysis. Br J Haematol 2018;182:916-20.
61. Jung KS, Cho SH, Kim SJ, Ko YH, Kang ES, Kim WS. L-asparaginase-based regimens followed by allogeneic hematopoietic stem cell transplantation improve outcomes in aggressive natural killer cell leukemia. J Hematol Oncol 2016;9:41.
62. Takahashi H, Sakai R, Hattori Y, et al. Successful disease control with L-asparaginase monotherapy for aggressive natural killer cell leukemia with severe hepatic failure. Leuk Lymphoma 2013;54:662-4.
63. Teshima T, Miyaji R, Fukuda M, Ohshima K. Bone-marrow transplantation for Epstein-Barr-virus-associated natural killer cell-large granular lymphocyte leukaemia. Lancet 1996;347:1124.
64. Murashige N, Kami M, Kishi Y, et al. Allogeneic haematopoietic stem cell transplantation as a promising treatment for natural killer-cell neoplasms. Br J Haematol 2005;130:561-7.
65. Ito T, Makishima H, Nakazawa H, et al. Promising approach for aggressive NK cell leukaemia with allogeneic haematopoietic cell transplantation. Eur J Haematol 2008;81:107-11.
66. Hamadani M, Kanate AS, DiGilio A, et al. Allogeneic hematopoietic cell transplantation for aggressive NK cell leukemia. A center for international blood and marrow transplant research analysis. Biol Blood Marrow Transplant 2017;23:853-6.
67. Jeong SH, Song HN, Park JS, et al. Allogeneic stem cell transplantation for patients with natural killer/T cell lymphoid malignancy: a multicenter analysis comparing upfront and salvage transplantation. Biol Blood Marrow Transplant 2018;24:2471-8.
68. Fujimoto A, Ishida F, Izutsu K, et al. Allogeneic stem cell transplantation for patients with aggressive NK-cell leukemia. Bone Marrow Transplant 2021;56:347-56.
69. Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res 2013;19:3462-73.
70. Kataoka K, Miyoshi H, Sakata S, et al. Frequent structural variations involving programmed death ligands in Epstein-Barr virus-associated lymphomas. Leukemia 2019;33:1687-99.
71. Kwong YL, Chan TSY, Tan D, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood 2017;129:2437-42.
72. Lim JQ, Huang D, Tang T, et al. Whole-genome sequencing identifies responders to Pembrolizumab in relapse/refractory natural-killer/T cell lymphoma. Leukemia 2020;34:3413-9.
73. Li X, Cheng Y, Zhang M, et al. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J Hematol Oncol 2018;11:15.
74. Chan TSY, Li J, Loong F, Khong PL, Tse E, Kwong YL. PD1 blockade with low-dose nivolumab in NK/T cell lymphoma failing L-asparaginase: efficacy and safety. Ann Hematol 2018;97:193-6.
75. Li J, Tao R, Fan L, et al. Sintilimab for relapsed/refractory (r/r) extranodal NK/T cell lymphoma (ENKTL): extended follow-up on the multicenter, single-arm phase II trail (ORIENT-4). J Clin Oncol 2020;38:8050.
76. Kim SJ, Lim JQ, Laurensia Y, et al. Avelumab for the treatment of relapsed or refractory extranodal NK/T-cell lymphoma: an open-label phase 2 study. Blood 2020;136:2754-63.
77. Gao Y, Huang H, Wang X, et al. Anti-PD-1 antibody (Sintilimab) plus histone deacetylase inhibitor (Chidamide) for the treatment of refractory or relapsed extranodal natural killer/T cell lymphoma, nasal type (r/r-ENKTL): preliminary results from a prospective, multicenter, single-arm, phase Ib/II trial (SCENT). Blood 2020;136:39-40.
78. Cai J, Liu P, Huang H, et al. Combination of anti-PD-1 antibody with P-GEMOX as a potentially effective immunochemotherapy for advanced natural killer/T cell lymphoma. Signal Transduct Target Ther 2020;5:289.
79. Du L, Zhang L, Li L, et al. Effective treatment with PD-1 antibody, chidamide, etoposide, and thalidomide (PCET) for relapsed/refractory natural killer/T-cell lymphoma: a report of three cases. Onco Targets Ther 2020;13:7189-97.
80. Kim HK, Moon SM, Moon JH, Park JE, Byeon S, Kim WS. Complete remission in CD30-positive refractory extranodal NK/T-cell lymphoma with brentuximab vedotin. Blood Res 2015;50:254-6.
81. Poon LM, Kwong YL. Complete remission of refractory disseminated NK/T cell lymphoma with brentuximab vedotin and bendamustine. Ann Hematol 2016;95:847-9.
82. Kim SJ, Yoon DH, Kim JS, et al. Efficacy of brentuximab vedotin in relapsed or refractory high-CD30-expressing non-Hodgkin lymphomas: results of a multicenter, open-labeled phase II trial. Cancer Res Treat 2020;52:374-87.
83. Hari P, Raj RV, Olteanu H. Targeting CD38 in refractory extranodal natural killer cell-T-cell lymphoma. N Engl J Med 2016;375:1501-2.
84. Huang H-q, Kim W-S, Yao M, et al. Daratumumab monotherapy for patients with relapsed or refractory (R/R) natural killer/T-cell lymphoma (NKTCL), nasal type: updated results from an open-label, single-arm, multicenter phase 2 study. Blood 2019;134:1568.
85. Shi Y, Dong M, Hong X, et al. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol 2015;26:1766-71.
86. Fujimoto A, Suzuki R. Epstein-Barr virus-associated post-transplant lymphoproliferative disorders after hematopoietic stem cell transplantation: pathogenesis, risk factors and clinical outcomes. Cancers (Basel) 2020;12:328.
87. Bollard CM, Gottschalk S, Torrano V, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol 2014;32:798-808.
88. Cho SG, Kim N, Sohn HJ, et al. Long-term outcome of extranodal NK/T cell lymphoma patients treated with postremission therapy using EBV LMP1 and LMP2a-specific CTLs. Mol Ther 2015;23:1401-9.
89. Huang Y, de Reyniès A, de Leval L, et al. Gene expression profiling identifies emerging oncogenic pathways operating in extranodal NK/T-cell lymphoma, nasal type. Blood 2010;115:1226-37.
90. Lee S, Park HY, Kang SY, et al. Genetic alterations of JAK/STAT cascade and histone modification in extranodal NK/T-cell lymphoma nasal type. Oncotarget 2015;6:17764-76.
91. Küçük C, Jiang B, Hu X, et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from γδ-T or NK cells. Nat Commun 2015;6:6025.
92. Nairismägi ML, Gerritsen ME, Li ZM, et al. Oncogenic activation of JAK3-STAT signaling confers clinical sensitivity to PRN371, a novel selective and potent JAK3 inhibitor, in natural killer/T-cell lymphoma. Leukemia 2018;32:1147-56.
93. Koo GC, Tan SY, Tang T, et al. Janus kinase 3-activating mutations identified in natural killer/T-cell lymphoma. Cancer Discov 2012;2:591-7.
94. Sim SH, Kim S, Kim TM, et al. Novel JAK3-activating mutations in extranodal NK/T-cell lymphoma, nasal type. Am J Pathol 2017;187:980-6.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Fujimoto A, Suzuki R. Progress in the treatment of NK-cell lymphoma/leukemia. J Cancer Metastasis Treat 2021;7:66. http://dx.doi.org/10.20517/2394-4722.2021.157
AMA Style
Fujimoto A, Suzuki R. Progress in the treatment of NK-cell lymphoma/leukemia. Journal of Cancer Metastasis and Treatment. 2021; 7: 66. http://dx.doi.org/10.20517/2394-4722.2021.157
Chicago/Turabian Style
Fujimoto, Ayumi, Ritsuro Suzuki. 2021. "Progress in the treatment of NK-cell lymphoma/leukemia" Journal of Cancer Metastasis and Treatment. 7: 66. http://dx.doi.org/10.20517/2394-4722.2021.157
ACS Style
Fujimoto, A.; Suzuki R. Progress in the treatment of NK-cell lymphoma/leukemia. J. Cancer. Metastasis. Treat. 2021, 7, 66. http://dx.doi.org/10.20517/2394-4722.2021.157
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 6 clicks
Cite This Article 6 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.