Cancer field surgery for locoregional tumor control of cervical carcinoma
Abstract
As the current standard, surgery is applied to treat early-stage cervical cancer and selected post-irradiation pelvic relapses. Surgical therapy for local disease is based on a model of unlimited isotropic cancer cell propagation and dissection artifacts such as subperitoneal “ligaments” and “spaces”. For regional disease, the role of traditional surgery is diagnostic and eventually cytoreductive. However, the isotropic local tumor propagation model has to be rejected due to numerous inconsistencies with clinical facts. Likewise, the “ligament and space” approach to the subperitoneum is too crude and variable to accurately cover both local spread and intercalated lymph node metastases of cervical cancer. The ontogenetic cancer field model is fully in line with the locoregional spread patterns of carcinoma of the female genital tract. Developmentally derived (ontogenetic) anatomy enables unbiased and accurate dissection of the complex tissue structures within the subperitoneum. Cancer field surgery founded on these insights has a high potential to improve the treatment outcome of cervical carcinoma.
Keywords
SURGICAL TREATMENT OF CERVICAL CARCINOMA: CURRENT STANDARD
The International Federation of Gynecology and Obstetrics (FIGO) revised the staging system of cervical cancer in 2018, integrating modern imaging techniques (e.g., MRI) into diagnostics and allowing for newer treatment options[1]. Early disease corresponds to stages IA1, IA2, IB1, IB2, and IIA1. Stages IIA2, IIB, IIIA, IIIB, and IVA comprise locally advanced disease; the state of IB3 cancer is debated. Stages IIIC1 and IIIC2 designate cervical cancer with regional (pelvic and periaortic) lymph node metastases irrespective of the local tumor extent (except for stage IVA).
Surgical treatment is restricted to early disease and may allow fertility preservation for the earliest stages up to IB1. All other stages of primary cervical cancer should be subjected to chemoradiation following the current treatment guidelines. To accurately diagnose the nodal stage, lymph node dissection preferably by minimal invasive techniques is recommended. The potential of sentinel lymph node biopsies to substitute for complete lymph node dissection is currently under investigation by randomized prospective trails (e.g., SENTICOL III, NCT 03386734).
Standard therapy for stages IA2-IIA1 after demonstrating the absence of lymph node metastases is radical hysterectomy. This time-honored operation aims to completely resect the local tumor and detect clinically occult cancer as well as intercalated lymph node metastases in the pericervical tissue (“parametrium”). Radical hysterectomy is a wide extirpation of the uterine cervix based on a surgical anatomy that subdivides the subperitoneal fibrofatty tissues into “spaces” and “ligaments”[2]. The operation follows the principle of unlimited isotropic local tumor propagation driven by undirected migration of cancer cells or cell clusters without respecting tissue borders.
However, the isotropic model is inconsistent with clinical facts and therefore falsified. As a logical consequence of the isotropic model, the width of the resection margin should predict the probability of local tumor recurrence. However, the significance of the margin width for the prevention of local relapses could not be substantiated despite numerous attempts in gynecologic oncology[3]. Anisotropic local spread of cervical cancer depending on the pericervical tissue could be directly demonstrated by color-coded tumor propagation landscapes[4]. The subperitoneal surgical anatomy of traditional radical hysterectomy is a crude simplification of the real pelvic anatomy. The “ligaments” and “spaces” are pure dissection artifacts, variable and biased by the surgeon performing the operation. The discrepancy with the architecture of the unmanipulated subperitoneal fibrofatty tissues is evident from the real and radiologic (MRI) sectional anatomy of the female pelvis. An operation founded on inconsistent tumor biological and anatomical conceptions is rather unlikely to bring forth optimal treatment results. This is verified by the high probability of locoregional relapses in early cervical cancer associated with histopathological risk factors, such as lymph node metastases and histopathological T2b stage demanding for postoperative adjuvant chemo-radiotherapy[5].
Fertility preserving surgical treatment has been shown to be oncologically safe in selected cases of early cervical cancer stages IA1-IB1. Following minimally invasive lymph node assessment, local therapy is performed by radical trachelectomy or conization[6,7]. The subperitoneal surgical anatomy of radical trachelectomy corresponds to that of conventional radical hysterectomy and is therefore also subjected to the criticism stated above.
At the other end of the current surgical treatment spectrum for cervical cancer, pelvic exenteration has been established for post-irradiation local relapses[8]. Multivisceral resection is founded on the same principles as radical hysterectomy, namely the isotropic local tumor spread model and the “spaces and ligaments” approach towards the subperitoneum. The goal of R0 resection is quite often not reached, which becomes obvious by reported abortion rates of 33%-52% and R1/2 resection rates of 8%-25%. Consequently, the indication for this mega-operation is limited to recurrent tumors located centrally in the pelvis[9].
THE ONTOGENETIC CANCER FIELD MODEL: LOCOREGIONAL CANCER FIELDS OF CERVICAL CARCINOMA
The ontogenetic cancer field model links the formation of malignant neoplastic tissue, i.e., the oncomorphogenesis, to the morphogenesis of metazoic development[10,11]. The formation of the animal organism from the fertilized egg is an extremely complex process that involves both deterministic and emergent morphogenetic information implemented in successive developmental phases[12]. The deterministic information relates to the cell type and function, its positional identity and plasticity, and is hierarchically stored by epigenetic mechanisms during the stepwise specification of each cell line to maturity. We postulate that this information is reactivated in adult cells transformed to malignancy by driver mutations and that the sequence of deterministic morphogenetic information is reversed during cancer progression. The positional identity and plasticity of the normal precursor cell of a distinct developmental state thus determines the permissive tissue for the cancer cell derived from the corresponding cell line at its distinct state of malignant progression.
According to the ontogenetic cancer field model, the local cancer fields also set up the regional cancer fields by peripheral antigens representing the positional identity of the tributary tissue presented in the regional lymph nodes. This positional information instrumental for peripheral immune tolerance and lymphocyte homing as mechanisms of the adaptive immune system is postulated to be abused by cancer cells for colonizing the regional secondary lymphoid tissue. In addition, taking into account the development of the lymphatic system from topographically defined lymph sacs, we deduced first-, second-, and third-line lymph node regions as regional cancer fields for any local cancer field of cervical carcinoma[13]. The validity of the ontogenetic cancer field model has been demonstrated from the local and regional spread patterns of more than 700 cases of cancer of the lower female genital tract[13,14]. It represents a principle of order (“order of cancer”) that can be exploited for diagnostics and treatment of solid malignant neoplasms by means of local and nodal ontogenetic staging and cancer field surgery.
To identify the local and regional cancer fields for carcinoma of the uterine cervix, the development of the mature cervical epithelium starting with the nephrogenic cords of the intermediate mesoderm at the phylotypic period (Carnegie stage 11) is followed topographically in the female. The mature tissue derivatives of the morphogenetic units comprised of the precursor cell populations and all adjacent cell populations with transdifferentiation potential, the Müllerian compartment, and the cervix subcompartment are identified and indicated in the topographic anatomic depiction of the pelvis by color coding. This ontogenetic anatomic map represents the local cancer fields of the stepwise malignant progression [Figure 1]. The ontogenetic stage (oT1-4) of the disease is determined by the clinical or histopathological allocation of the malignant cells or tissue to a cancer field. The topography of the regional cancer fields for cervical carcinoma is deduced from the architecture of lymphatics draining the local cancer fields and the development of the lymphatic system from the iliacal, lumbar, and mesenteric lymph sacs. Metastatic colonization of the first-, second-, or third-line lymph node regions indicates the ontogenetic nodal stage (oN1-3), providing important information of the overall regional metastasis pattern to be expected. In our patient cohort, both oT and oN stages of cervical carcinoma have been proven to be superior to conventional clinical or histopathological classification in predicting the disease outcome[13,15].
Figure 1. Local cancer fields for carcinoma of the uterine cervix determined by development. (A) Lineage specification pathway of cervical epithelial and stromal cells from nephrogenic cord precursor cells. (B) Ontogenetic anatomy of the uterine cervix displayed in a midpelvic transverse section as indicated by the sagittal inset. Mature tissue derivatives from the morphogenetic units of the three generations of precursor cells are coded light, middle, and dark grey, respectively. Mature derivatives of the Müllerian compartment and its cervical subcompartment are colored light and dark green, respectively. The color code corresponds to (A). (C) Local cancer fields of cervical carcinoma depicted as red areas in the same midpelvic plane as in (B) for stepwise malignant progression of the transformed cells from ontogenetic stage oT1 to oT4. Modified from Höckel et al.[15].
CANCER FIELD SURGERY FOR CERVICAL CARCINOMA: TMMR, EMMR, LEER, AND THERAPEUTIC LYMPH NODE DISSECTION
Cancer field surgery and therapeutic lymph node dissection are the therapeutical translation of the ontogenetic cancer field model for epithelial malignant tumors, i.e., carcinomas. As isotropic microscopic and occult cancer propagation is assumed within the cancer field, local tumor control demands its complete resection. For tumors of small volume relative to the size of the cancer field, wide excision within the cancer field can be sufficient. At the border of the cancer field, local control is achieved with narrow resection margins which can significantly reduce treatment-related morbidity. For the execution of cancer field surgery, optimal preoperative diagnosis, particularly high-resolution pelvic MRI, and intraoperative assessment of distinct tissue sites (e.g., uterine corpus) and resection margins (e.g., within the cancer field) by the pathologist are essential. Likewise, frozen section evaluation of distinct lymph node dissection is mandatory according to validated algorithms for regional tumor control. This practice following the principles of cancer field surgery allows to completely wave adjuvant radiotherapy for locoregional tumor control even in high-risk cases.
With respect to the locoregional cancer fields of cervical carcinoma, the following surgical techniques have been developed and validated: total mesometrial resection (TMMR)[13] extended mesometrial resection (EMMR)[16], and laterally extended endopelvic resection (LEER)[17]. Each of them is combined with therapeutic pelvic and periaortic lymph node dissection (tLND).
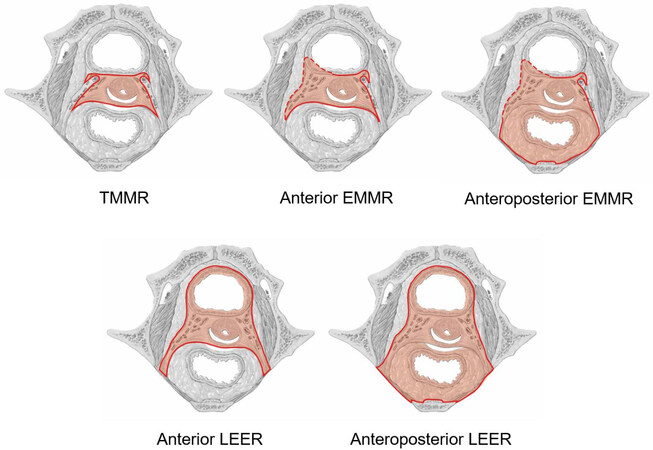
Figure 2 provides an overview of the resected tissues by the different procedures in a midpelvic transverse section. Compared to the ontogenetic anatomy depicted in the same pelvic plane, it is obvious that the ontogenetic cancer fields of cervical carcinomas up to oT3b are still completely resectable, whereas only parts of the oT4 field can be surgically removed. This statement holds true for the 3D pelvis (not shown). Therapeutic lymph node dissection has to be adapted to the local and nodal ontogenetic stage, as illustrated below.
Figure 2. Survey of cancer field operations for the treatment of cervix carcinoma. Resection lines and resected tissues shown in the same midpelvic plane as in Figure 1 are colored dark and light red, respectively. TMMR: Total mesometrial resection; EMMR: extended mesometrial resection; LEER: laterally extended endopelvic resection.
Total mesometrial resection was designed for the locoregional control of cervical carcinoma of the ontogenetic stages oT1 and oT2 and all associated nodal stages, oN0 and oN1-3. Therefore, TMMR treats all pT stages 1b1, 2, 2a1, 2, and a significant percentage of pT2b stages. In terms of the 2018 FIGO classification, stages IB1, 2, 3, IIA1, 2, the majority of IIB stages (64% in our series), and IIIC1, 2 of oT1, 2 local extent are suitable for treatment by TMMR. TMMR extirpates the complete Müllerian compartment except more distal parts of the vagina, which are preserved for function. Therefore, a wide vaginal resection margin is mandatory in oT2 cases. TMMR also includes the complete vascular mesometrium and associated parts of the ligamentous mesometrium. These non-Müllerian tissues defined by ontogenetic anatomy contain intercalated first-line lymph nodes and may harbor both lymph node metastases and tumor cell clusters in transit. Thus, part of the therapeutic lymph node dissection is performed with the TMMR. A major distinction from the traditional radical hysterectomy, which removes formal proximo-distal parts of the “parametrium” as a tissue unit, TMMR respects nine different paracervical tissue structures defined by the morphogenesis of the Müllerian system. Four of these tissues are resected, while five are preserved irrespective of tumor size. Characteristic features of the procedure, which is described step-by-step with high-resolution surgical anatomy, are: (1) the dissection along developmentally determined surgical planes; and (2) the transection of tissue structures which have been completely exposed before[13,18].
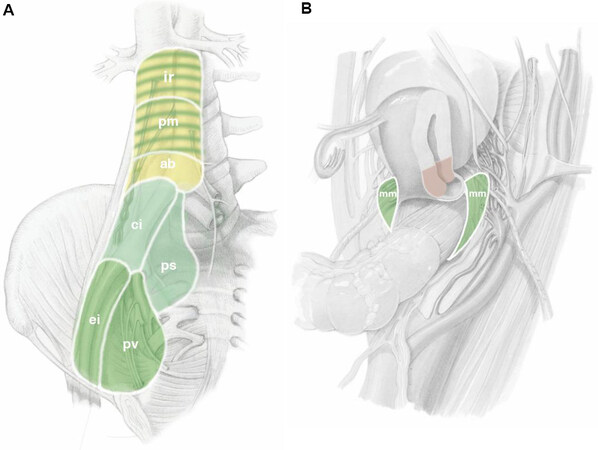
Therapeutic resection of the basin lymph nodes according to the case-specific algorithm completely removes the lympho-fatty tissues of the first-, second-, and third-line regions within the pelvic and periaortic retroperineum [Figure 3]. To achieve this surgical goal for regional tumor control, parietal rami of the internal iliac vessels and the vena cava have to be sealed and severed, exposing the proximal sciatic nerves and the anterior longitudinal ligament of the spine. All autonomous nerves essential for the function of the remaining pelvic organs are preserved. The mature oncological results of TMMR and therapeutic lymph node dissection obtained from the prospective unicentric Leipzig School observational cohort study are strikingly superior to those of the standard treatment of radical hysterectomy and adjuvant
Figure 3. Regional cancer fields for cervix carcinoma. First-, second-, and third-line lymph node regions are colored dark green, light green, and yellow, respectively. Cervical cancers invading the uterine corpus exhibit first-line lymph node regions both in the pelvis and in the mesenteric periaortic region (dark green stripes). (A) parietal node regions; (B) intercalated node regions. ei: External iliac; pv: paravisceral; ci: common iliac; ps: presacral; ab: aortic bifurcation; pm: perimesenteric; ir: infrarenal; mm: meosmetrial. From
Modifying TMMR into a fertility preserving procedure retaining the uterine corpus and anastomosing it to the vaginal stump is technically feasible. However, the concept of ontogenetic cancer fields argues against a fertility preserving mesometrial resection of low-volume, low-risk cervical carcinoma. From the clinical and histopathological results of the prospective Leipzig School observational cohort study on cancer field surgery for cervical carcinoma, it can be concluded that: (1) the risk of local recurrence from occult tumor spread into the paracervical tissues and the vagina is much lower than that from occult cancer in the subisthmic cervical tissue that has to be retained to achieve acceptable obstetrical outcomes; and (2) the risk of recurrence from undetected intercalated mesometrial lymph node metastases may be around 1%. It is therefore questionable whether the theoretical risk reduction by “radical trachelectomy” as compared to conization in FIGO IB1, L0, pN0 cervical cancer can be statistically proven. Evidence from multivariate analysis of a recent multi-institutional retrospective review study of cervical cancer FIGO stages IA1-IB1, ≤ 2 cm tumor size[19] and from sarcoma surgery[20] indicate a significant risk reduction of recurrence from occult cancer cells by primary tumor resection followed by a secondary resection of the previous tumor periphery (“tumor bed”). The fertility-preserving concept for the surgical treatment from a cancer field view is a minimally invasive surgical assessment of the nodal state after a first conization allowing precise histopathological evaluation followed by a tailored secondary conization after six weeks for cervical carcinoma sized < 2 cm, L0, pN0.
Cancer field surgical procedures for locally advanced and recurrent cervical carcinoma include EMMR and LEER to be performed in multiple variants. Whereas EMMR procedures preserve urinary and intestinal pelvic functions, those have to be substituted with LEER. According to the ontogenetic cancer field model, the locally advanced state begins at the ontogenetic stage oT3a [Figure 1] when the tissues permissive for local tumor propagation exceed the developmentally defined mature compartment of the cell line.
Anterior EMMR may include the unilateral bladder mesentery, the ipsilateral distal ureter, and parts of the bladder necessitating ureterovesiconeostomy. Posterior EMMR is performed by resecting the ligamentous mesentery together with a segment of the mesorectum and associated rectum. End-to-end anastomosis restores the bowel continuity. EMMR extirpates part of but not the complete oT3a cancer field. We demonstrated with 99 successive patients being diagnosed with unselected FIGO IIB carcinomas that R0 resection was achieved with either TMMR (n = 49) or EMMR (n = 50) including therapeutic lymph node dissection according to the cancer field algorithm for regional tumor control. Whether treatment of FIGO IIB cervical carcinoma by cancer field surgery may be a potential alternative for (chemo-)radiotherapy is currently under investigation at the Leipzig School (DRKS 00015171). The treatment-related morbidity of EMMR is about twice as high as that of TMMR for FIGO IB1 and IIA cases. Moderate and severe complications occurred in 40%. However, with TMMR for FIGO IIB tumor morbidity is also increased[13,16].
Laterally extended endopelvic resection is mainly carried out as anterior or total procedure. Indications for a posterior LEER are rare. Anterior LEER is necessary to remove the complete oT3a cancer field; the resection of oT3b demands the total procedure as the complete pelvic autonomous nerves belong to the permissive tissues. Although parts of the oT4 cancer field can be included in the total LEER, its complete resection is impossible as the oT4 cancer field contains most of the tissues of the lower trunk. The technical difference of LEER as cancer field surgery from pelvic exenteration is the complete inclusion of the pelvic subperitoneum in the multivisceral resection by the former. To accomplish this, the internal iliac vessel system and the pelvic floor muscles are part of the LEER specimen. Treatment-related morbidity is significant with 45% moderate and 9% severe events[17], particularly due to the extensive surgical reconstruction or substitution of the vital pelvic functions (micturition, defecation, pelvic floor, and perineum).
LEER is indicated as potential salvage treatment in situations without other therapeutic options such as post-irradiation local recurrences of cervical carcinoma fixed to the pelvic sidewall. Candidate patients for LEER treatment must have no evidence for distant metastases. Contraindications with regard to the pelvic disease are multiple tumors and involvement of the somatic pelvic tissues (muscle, bone, large vessels, and nerves). In our series of 54 successive patients with mainly post-irradiation pelvic relapses fixed to the pelvic wall, all tumors were resected with clear margins. Five-year overall survival was 46% (95%CI: 34%-62%)[17].
CONCLUSION AND PERSPECTIVES
The ontogenetic cancer field model provides a principle of order concerning the malignant progression of the transformed cells that can be clinically exploited for diagnosis, therapy, and prognosis. Moreover, the model explains shortcomings and inconsistencies of current principles and practice such as the missing robustness of margin width with respect to predicting local relapses.
The Leipzig School of Radical Pelvic Surgery has demonstrated the high potential of cancer field surgery to improve the treatment results for carcinoma of the uterine cervix and the vulva. The ontogenetic field model also elucidates the general limits of the surgical treatment of locally advanced disease as the extension of the tissues permissive for cancer propagation exceeds their resectability with acceptable consequences when the cancer cells have progressed to states before determination (> oT2). Certainly, the proof of reproducibility of both the oncological results and treatment-related morbidity achieved by the Leipzig School by prospective multicenter studies is mandatory. The report of first preliminary data of the European TMMR register study is, however, very promising[21]. Of 116 patients with cervical cancer stages IB-IIA, (FIGO 2018) who underwent TMMR/tLND, 25.0% were lymph node positive. pT stages were pT1a in 3 patients (2.6%), pT1b1 in 82 (70.7%), pT1b2 in 18 (15.5%), pT2a in 4 (3.5%), and pT2b in 9 (7.8%). The overall recurrence rate was 7.8% in a median follow-up time of 24 months (6-80 months). Locoregional recurrences occurred in 6.0% of patients. One patient (0.9%) died from the disease during the observation period.
It should also be explored whether treatment based on the ontogenetic cancer field model may improve the outcome of other malignant solid tumors in gynecologic oncology (e.g., endometrial cancer) as well as non-gynecologic oncology. Generally, the maximum benefits of cancer field surgery are expected if the disease is detected in the oT1 and oT2 stages.
Finally, research addressing the biological mechanisms dictating cancer cell propagation within distinct developmentally determined tissues is exciting. Investigating the epigenetic processes responsible both for the stepwise morphogenesis and, in reverse sequence, for the oncomorphogenesis during malignant progression appears to be very rewarding.
DECLARATIONS
Authors’ contributionsThe author contributed solely to the article.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestThe author declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2021.
REFERENCES
1. Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet 2019;145:129-35.
3. Höckel M, Horn LC. The puzzle of close surgical margins is not puzzling. Gynecol Oncol 2013;130:224-5.
4. Kubitschke H, Wolf B, Morawetz E, et al. Roadmap to local tumour growth: insights from cervical cancer. Sci Rep 2019;9:12768.
6. Segarra-Vidal B, Persson J, Falconer H. Radical trachelectomy. Int J Gynecol Cancer 2021;31:1068-74.
7. Nica A, Marchocki Z, Gien LT, et al. Cervical conization and lymph node assessment for early stage low-risk cervical cancer. Int J Gynecol Cancer 2021;31:447-51.
9. Höckel M, Dornhöfer N. Pelvic exenteration for gynaecological tumours: achievements and unanswered questions. Lancet Oncol 2006;7:837-47.
10. Höckel M. Morphogenetic fields of embryonic development for locoregional cancer spread. Lancet Oncol 2015;16:e148-51.
11. Höckel M, Behn U. The order of cancer: a theory of malignant progression by inverse morphogenesis. Front Oncol 2019;9:416.
12. Collinet C, Lecuit T. Programmed and self-organized flow of information during morphogenesis. Nature Rev Mol Cell Biol 2021;22:245-65.
13. Höckel M, Wolf B, Schmidt K, et al. Surgical resection based on ontogenetic cancer field theory for cervical cancer: mature results from a single-centre, prospective, observational, cohort study. Lancet Oncol 2019;20:1316-26.
14. Höckel M, Trott S, Dornhöfer N, Horn L, Hentschel B, Wolf B. Vulvar field resection based on ontogenetic cancer field theory for surgical treatment of vulvar carcinoma: a single-centre, single-group, prospective trial. Lancet Oncol 2018;19:537-48.
15. Höckel M, Hentschel B, Horn L. Association between developmental steps in the organogenesis of the uterine cervix and locoregional progression of cervical cancer: a prospective clinicopathological analysis. Lancet Oncol 2014;15:445-56.
16. Wolf B, Ganzer R, Stolzenburg JU, Hentschel B, Horn LC, Höckel M. Extended mesometrial resection (EMMR): surgical approach to the treatment of locally advanced cervical cancer based on the theory of ontogenetic cancer fields. Gynecol Oncol 2017;146:292-8.
17. Höckel M, Wolf B, Hentschel B, Horn LC. Surgical treatment and histopathological assessment of advanced cervicovaginal carcinoma: a prospective study and retrospective analysis. Eur J Cancer 2017;70:99-110.
18. Höckel M, Horn L, Fritsch H. Association between the mesenchymal compartment of uterovaginal organogenesis and local tumour spread in stage IB-IIB cervical carcinoma: a prospective study. Lancet Oncol 2005;6:751-6.
19. Uppal S, Gehrig PA, Peng K, et al. Recurrence rates in patients with cervical cancer treated with abdominal versus minimally invasive radical hysterectomy: a multi-institutional retrospective review study. J Clin Oncol 2020;38:1030-40.
20. Lewis JJ, Leung D, Espat J, Woodruff JM, Brennan MF. Effect of reresection in extremity soft tissue sarcoma. Ann Surg 2000;231:655-63.
21. Buderath P, Stukan M, Ruhwedel W, et al. Total mesometrial resection (TMMR) for cervical cancer FIGO IB-IIA. First results from the multicentric TMMR register study. J Gynecol Oncol .
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Höckel M. Cancer field surgery for locoregional tumor control of cervical carcinoma. J Cancer Metastasis Treat 2021;7:64. http://dx.doi.org/10.20517/2394-4722.2021.145
AMA Style
Höckel M. Cancer field surgery for locoregional tumor control of cervical carcinoma. Journal of Cancer Metastasis and Treatment. 2021; 7: 64. http://dx.doi.org/10.20517/2394-4722.2021.145
Chicago/Turabian Style
Höckel, Michael. 2021. "Cancer field surgery for locoregional tumor control of cervical carcinoma" Journal of Cancer Metastasis and Treatment. 7: 64. http://dx.doi.org/10.20517/2394-4722.2021.145
ACS Style
Höckel, M. Cancer field surgery for locoregional tumor control of cervical carcinoma. J. Cancer. Metastasis. Treat. 2021, 7, 64. http://dx.doi.org/10.20517/2394-4722.2021.145
About This Article
Copyright
Data & Comments
Data

 Cite This Article 5 clicks
Cite This Article 5 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.