Biomarkers for therapy selection in metastatic urothelial cancer
Abstract
The treatment of metastatic urothelial cancer (mUC) has been transformed by recent progress in clinical trials and drug development. There are now three therapeutic classes with proven benefits in mUC: chemotherapy, immunotherapy, and targeted therapy. The optimal sequence and combination of these classes remain to be defined. Biomarker development is essential to guide treatment selection at each therapeutic juncture. Two biomarkers, programmed death-ligand 1 expression and fibroblast growth factor receptor alterations, have been incorporated into the mUC treatment paradigm thus far. This review discusses predictive biomarkers in development and their potential to influence mUC treatment selection moving forward.
Keywords
INTRODUCTION
For 30 years, cisplatin-based combination chemotherapy was the only treatment to demonstrate a meaningful overall survival (OS) benefit in metastatic urothelial cancer (mUC)[1]. In subsequent lines, patients were treated with single-agent chemotherapy with limited benefit[1]. This sparse treatment landscape has been transformed by contemporary trials demonstrating OS benefits from anti-
The expansion of mUC treatment options raises the question of treatment selection. How can therapeutic options be prioritized to maximize benefits and minimize harms for individual patients? One answer to this question is: biomarkers. Ideally, biomarkers guide clinical management by clearly distinguishing patients who will or will not benefit from treatment. This review will discuss molecular biomarkers in clinical use and in development for treatment selection in metastatic urothelial cancer.
BIOMARKER USES AND DEVELOPMENT
While a comprehensive description of biomarker types and their development is outside the scope of this review, a brief overview may be useful to situate the various biomarkers described herein along the axis of predictive vs. prognostic and along the continuum from exploratory to clinically validated.
A biomarker can be predictive, prognostic, or both. Predictive biomarkers are associated with outcomes in the context of specific treatments, while prognostic biomarkers are associated with outcomes irrespective of treatment. This treatment-dependent distinction implies that a biomarker’s classification may change as a disease’s treatment landscape evolves. This is exemplified by the transformation of human epidermal growth factor receptor 2 (HER2) amplification in breast cancer from an adverse prognostic marker into a treatment-defining predictive marker with the advent of effective anti-HER2 agents. Today’s prognostic markers may be tomorrow’s drug targets.
As this review is concerned with biomarkers that can guide treatment selection, our focus is on predictive biomarkers. Aside from two predictive biomarkers routinely used in mUC management - PD-L1 and FGFR alterations - the other candidate biomarkers discussed here have yet to be prospectively validated for treatment selection. These candidate biomarkers typically have demonstrated associations with outcomes in retrospective cohorts or post-hoc analyses of clinical trials and may have in vitro data to support a mechanistic link between the biomarker and treatment outcomes. Where possible, we will highlight biomarkers being incorporated into prospective trials, as these have the greatest potential to become clinically validated.
To formally establish a biomarker as predictive, there should be a comparison of outcomes by both treatment arm (experimental vs. control) and biomarker status (positive or negative). Without a control arm, it is not possible to determine whether the biomarker’s association with outcomes is specific to the experimental treatment (predictive) or a general phenomenon (prognostic). That said, in some cases, the mechanistic link between the predictive biomarker and the treatment’s mechanism is seen as strong enough to justify running a prospective trial in a biomarker-selected population, effectively assuming that there would be no benefit from the treatment in a biomarker-negative population.
The treatment landscape, the mechanistic link between biomarker and treatment, and the level of evidence available should all be considered when evaluating the clinical utility of a novel biomarker.
CHEMOTHERAPY BIOMARKERS
Cisplatin-based combination chemotherapy regimens are the backbone of first-line mUC treatment. Randomized clinical trials published in the 1990s demonstrated that the combination of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) improved OS compared to cisplatin alone and compared to the combination of cisplatin, cyclophosphamide, and doxorubicin[8,9]. Subsequently, the combination of gemcitabine and cisplatin (GC) was shown to have similar survival outcomes as MVAC, with a more favorable safety profile[10].
At present, despite the introduction of novel agents for mUC, cisplatin-based chemotherapy remains the standard front-line treatment for advanced UC in patients without contraindications to it [Figure 1]. Immunotherapy has a role as maintenance treatment after chemotherapy[2], but upfront chemo-immunotherapy combinations and immunotherapy-only combinations have not shown OS benefits compared to chemotherapy alone[11-13]. The chemotherapy-free regimen of enfortumab vedotin plus pembrolizumab (EV/P) has shown promising early-phase results in the front-line cisplatin-ineligible setting[14] and is being tested against upfront chemotherapy in an unselected mUC population in the EV-302 phase III trial (NCT04223856). In this evolving treatment landscape, chemotherapy biomarkers would be valuable to distinguish responders - likely well-served by the current paradigm of upfront chemotherapy - from non-responders, who may benefit from novel combinations or alternative agents.
Figure 1. Current landscape. Current metastatic urothelial carcinoma (mUC) treatment landscape. Clinical factors which influence treatment selection, such as cisplatin-eligibility, are highlighted in green. Biomarkers are highlighted in yellow. Treatment options are shown in blue. In the second line, erdafitinib is an FDA-approved option but results from the phase III THOR trial (NCT03390504) comparing it against pembrolizumab are not yet available, thus it is shown with a dotted outline. PD-L1: Programmed death-ligand 1; ICI: immune checkpoint inhibitor; 1L: first-line; 2L: second-line; 3L: third-line.
It is worth noting that most of the data concerning chemotherapy biomarkers discussed below come from studies of muscle-invasive bladder cancer (MIBC). Neoadjuvant chemotherapy (NAC) regimens for MIBC are similar to regimens for mUC. However, until recently, a chemotherapy response biomarker was arguably more useful in the context of MIBC since the complete response to NAC has prognostic implications which may guide further treatment.
DNA damage repair pathways
Cisplatin binds to DNA, creating adducts and crosslinks that inhibit transcription and replication, and may lead to apoptosis. In normal cells, multiple DNA damage repair (DDR) pathways are activated in response to these lesions and cooperate to repair them[15]. These include nucleotide excision repair (NER), homologous recombination repair (HRR), and Fanconi anemia (FA) pathways. Mutations in each of these pathways, or in genes regulating these pathways, have been linked to platinum sensitivity in bladder cancer.
Nucleotide excision repair
The NER pathway recognizes bulky DNA adducts, such as those formed by cisplatin and ultraviolet radiation, and excises them[16]. Germline mutations in NER genes cause xeroderma pigmentosum, an autosomal recessive disorder characterized by marked photosensitivity. In fact, several NER genes (XPA-G) were initially named for their association with xeroderma pigmentosum. Just as germline mutations in NER genes can predispose individuals to UV sensitivity, tumor somatic mutations in NER pathway genes such as excision repair cross-complementation group 1 and 2 (ERCC1 and ERCC2) can also alter tumor cisplatin sensitivity.
ERCC1 expression levels
ERCC1 is a key NER gene linked to cisplatin sensitivity in a variety of solid tumors. The ERCC1 protein heterodimerizes with ERCC4 to form an endonuclease complex which participates in the excision of the damaged DNA. Lower ERCC1 levels, measured by mRNA expression or immunohistochemistry (IHC), have been correlated with cisplatin sensitivity in lung, cervical, ovarian, gastric, and colorectal cancers[17-22]. While most of these observations were made in small or retrospective cohorts, the IALT Bio study involved a cohort of 761 lung cancer patients who had participated in a prospective study of adjuvant cisplatin-based chemotherapy vs. observation. In this cohort, the authors found that the absence of ERCC1 staining by IHC was predictive of a benefit from adjuvant chemotherapy (test for interaction, P = 0.009)[19].
In urothelial carcinoma, low ERCC1 levels by either mRNA quantification or IHC have also been associated with improved response to cisplatin in the perioperative and metastatic settings[23-34]. In 2007,
ERCC2 mutations
ERCC2 is mutated in about 10% of muscle-invasive urothelial carcinomas[36]. It encodes a helicase subunit of the Transcription factor II Human complex, which plays a key role in the NER pathway by unwinding DNA at the site of damage prior to excision of the damaged bases. The majority of ERCC2 mutations in UC affect the helicase domain and in vitro data suggest that these mutations impair NER activity and confer cisplatin sensitivity[37,38].
Mutations in ERCC2 have been associated with complete pathological responses and improved OS after neoadjuvant cisplatin-based chemotherapy in two independent MIBC cohorts[38,39]. Similar, though non-significant, trends have been observed in other neoadjuvant MIBC cohorts[40-42]. In the metastatic setting, a recent study including a mix of patients with locally advanced (n = 62) and metastatic UC (n = 245) reported that the ERCC2-associated mutation signature SBS5, but not ERCC2 mutations, was correlated with response[43,44].
In the context of chemo-immunotherapy, the ongoing HCRN GU 16-257 study recently reported that ERCC2 mutations were associated with clinical and pathological complete response in MIBC patients treated with neoadjuvant gemcitabine, cisplatin, and nivolumab[45].
Homologous recombination
HRR is a high-fidelity DNA repair mechanism, which is involved in the repair of double-stranded breaks and interstrand crosslinks. In HRR, the undamaged homologous chromosome serves as a template for the repair of the damaged strand. BRCA1 and BRCA2 are perhaps the most widely recognized HRR genes, known for their roles as cancer predisposition genes and as predictive biomarkers for sensitivity to poly ADP-ribose polymerase (PARP) inhibitors and platinum-based chemotherapy.
The predictive association between BRCA1/2 mutations and platinum chemotherapy is best established in breast and ovarian cancer[46-48]. In breast cancer, the phase III TNT trial found a significant interaction between BRCA1/2 mutation status (either germline or somatic) and response to carboplatin; the objective response rate for carboplatin among BRCA-mutated tumors was twice that of BRCA-wild-type tumors (68% vs. 33%, P = 0.03)[48]. In ovarian cancer, the mechanistic link between BRCA2 deficiency and platinum sensitivity is illustrated by the observation that mutations which restore wild-type BRCA2 function also confer platinum resistance[47].
BRCA mutations can also be found in urothelial cancer and may be associated with platinum sensitivity. Somatic BRCA1/2 alterations were present in 19% of MIBC samples in TCGA (The Cancer Genome Atlas), and germline BRCA1/2 variants have been observed in 2%-4% of UC patients[36,49,50]. In a recent multi-omic analysis of 300 patients with MIBC or mUC, BRCA2 mutations were associated with the single base substitution 5 (SBS5) mutation signature and with chemotherapy response[43]. In contrast to BRCA2 mutations, BRCA1/2 expression levels have not been associated with outcomes in retrospective platinum-treated UC cohorts[34,51].
RAD51 (RAD51 recombinase) is another HRR gene, which is being investigated as a chemotherapy biomarker in UC. In the context of double-stranded breaks, the RAD51 protein (in conjunction with BRCA2) coordinates the process matching the damaged strands to their homologous complements[52]. High nuclear staining for RAD51 has been associated with worse OS for mUC patients treated with cisplatin-based chemotherapy[51].
Other DDR genes
Other DDR genes have been linked to cisplatin sensitivity. A 2015 study by Plimack et al.[53] used decision tree analysis to identify tumor mutations that could efficiently classify MIBC patients by cisplatin responsiveness. Out of 287 cancer-related genes on the sequencing panel, their analysis found that mutations in ATM (ATM serine/threonine kinase), RB1 (RB transcriptional corepressor 1), or FANCC (FA complementation group C) most clearly separated pathologic responders from non-responders in two clinical trial cohorts. An updated report showed that 5-year OS was 85% in those with ATM/RB1/FANCC mutations compared to 46% in those without[54]. ATM and RB1 are cell cycle regulators in response to DNA damage, while FANCC is a member of the core FA complex that is critical to interstrand crosslink repair.
Mutations in DDR genes as a class have also been associated with platinum response. In a 2017 retrospective analysis, Teo et al.[55] found that alterations in a panel of 34 DDR genes (representing various pathways) were associated with improved PFS and OS in platinum-treated mUC patients. The most commonly mutated DDR genes were ATM (n = 18), BRCA2 (n = 13), BRCA1 (n = 9), and NBN (n = 10), a component of the MRN complex that recognizes double-stranded breaks and initiates HRR.
Other somatic alterations
Groenendijk et al.[41] found ERBB2 (erb-b2 receptor tyrosine kinase 2) missense mutations (but not ERBB2 amplification) to be associated with response to platinum-based NAC in a retrospective study of 38 responding and 33 non-responding MIBC patients. There were 9 ERBB2 mutations among the responders and none among the non-responders (24% vs. 0%, P = 0.003). Van Allen et al.[38] had only 3 ERBB2-altered samples in their cohort, but all 3 were responders (P = 0.12). Conversely, Plimack et al.[53] did not find ERBB2 alterations to be associated with response, though they did not report separate analyses for ERBB2 missense mutations vs. amplification. Further analysis is required to determine whether ERBB2 missense mutations are a potential marker of chemosensitivity.
Molecular classifications
The extraction and quantification of tumor RNA enable tumors to be described by their gene expression profiles. This opens another avenue to differentiate tumors at the molecular level and potentially identify subgroups with differential response to antineoplastic agents. Clustering algorithms make it possible to group tumors into molecular subtypes based on similarities in gene expression.
Several efforts have been made to establish molecular taxonomies of UC, mostly based on nonmetastatic or MIBC samples[36,56-70]. However, the molecular subtypes generated by different groups can vary based on differences in cohorts, algorithms, and analytic decisions made by the investigators. Recently,
The distinction between luminal and basal UC is a recurring theme across molecular subtyping studies. These subtypes were initially named after their similarity to basal and luminal breast cancer subtypes[64,65,70]. Basal UC is associated with squamous differentiation and expression of basal cytokeratins such as KRT5, KRT6, and KRT14. The luminal subtype is characterized by the expression of KRT20 (keratin 20), GATA3 (GATA binding protein 3), and FOXA1 (forkhead box A1) and is associated with FGFR3 alterations and papillary histology. The consensus subtyping further divides luminal into three subtypes: luminal papillary, associated with a non-invasive papillary signature and FGFR3 transcriptional activity; luminal nonspecified, associated with increased fibroblastic infiltration; and luminal unstable, associated with genomic instability and increased cell cycle activity[71].
The utility of molecular subtypes as predictive biomarkers of chemotherapy response is unclear, and studies have produced conflicting results. Choi et al.[64] previously described a p53-like subtype that was chemo-resistant in small retrospective cohorts. This subtype was associated with the worst outcomes in a single-arm trial of neoadjuvant MVAC with bevacizumab[72]. The p53-like subtype falls under the stroma-rich consensus subtype, but none of the consensus subtypes were found to associate with NAC response in the Kamoun et al.[71] publication.
Some studies have suggested that basal UC is a chemo-sensitive subtype[64,72,73]. Seiler et al.[73] observed that patients with basal-type tumors had the greatest benefit from NAC compared to other subtypes, though they did not formally test for an interaction between NAC and subtype. The basal subtype was also associated with the best OS in the trial of neoadjuvant MVAC with bevacizumab mentioned above[72]. On the other hand, Taber et al.[43] recently reported that the basal/squamous consensus subtype was associated with reduced NAC response in a cohort of 300 advanced UC patients.
Another approach to developing gene expression-based predictive biomarkers, known as coexpression extrapolation (COXEN), has been proposed by Smith et al.[74]. The COXEN approach identifies gene expression signatures in cancer cell lines associated with in vitro chemotherapy sensitivity and extrapolates those signatures to predict chemosensitivity in vivo. COXEN gene expression models for response to cisplatin-based NAC regimens were tested in the phase II SWOG S1314 trial, but failed to predict response to either GC or ddMVAC[75].
Clinical trials examining chemotherapy biomarkers
The CALGB 90601 study (NCT00942331) was a phase III trial that enrolled 506 patients and compared gemcitabine and cisplatin with or without bevacizumab for the front-line treatment of mUC. There was no significant difference in the primary outcome of OS between the two arms[76]. However, the trial protocol also included correlative sub-studies aimed at testing potential chemotherapy biomarkers, including tumor expression of ERCC1, RAD51, RRM1 (ribonucleotide reductase catalytic subunit M1), BRCA1, BRCA2, and caveolin-1; somatic mutations in ERCC2; and the MD Anderson molecular subtypes (basal, luminal, p53-like)[77]. These results have yet to be reported.
Two ongoing phase II studies are testing biomarker-guided bladder-preservation strategies in MIBC treated with NAC (NCT03609216, NCT02710734). In these trials, patients with DDR mutations (e.g., ATM, RB1, FANCC, ERCC2) with complete clinical responses after neoadjuvant cisplatin-based chemotherapy can be actively monitored instead of undergoing cystectomy. An interim analysis of the RETAIN (Risk Enabled Therapy After Initiating Neoadjuvant Chemotherapy for Bladder Cancer) study reported that 33 of 71 patients had a DDR mutation of interest, with 28 opting for active surveillance[78]. In the HCRN GU 16-257 study, MIBC patients were treated with neoadjuvant gemcitabine, cisplatin, and nivolumab; ERCC2 mutations were associated with clinical and pathological complete response[45]. While these studies are in MIBC rather than mUC, positive data supporting a role for DDR genes as biomarkers in this setting could motivate efforts to define their role in treatment selection for mUC.
IMMUNOTHERAPY BIOMARKERS
ICIs have had a major impact on the treatment of advanced UC. Between 2016 and 2017, several early-phase clinical trials reported response rates of approximately 20% for single-agent anti-PD-1/PD-L1 in mUC[3,79-84]. Many randomized trials have followed, seeking to define the optimal role for single-agent ICIs and to test ICIs in combination with chemotherapy, targeted therapies, or other ICIs targeting different immune checkpoints.
At present, the strongest evidence for ICI benefit in mUC is in the post-platinum chemotherapy setting. Two randomized trials - KEYNOTE-045 and JAVELIN Bladder 100 - have demonstrated OS benefits for single-agent ICIs as either second-line or maintenance therapy after platinum chemotherapy[2,3]. Both trials met their primary OS endpoints in biomarker-unselected, all-comer populations. Notably, second-line ICI therapy was permitted in the control arm of JAVELIN Bladder 100 and was received by approximately one-third of control patients[85]. Consequently, in the post-platinum setting, maintenance ICI rather than ICI at progression is considered the preferred approach in NCCN (National Comprehensive Cancer Network) guidelines, though there may be a risk of overtreating some patients with this approach.
Exploratory analyses have found associations between higher tumor mutation burden (TMB) and PD-L1 expression with improved OS benefit from maintenance avelumab[86]. Gene expression signatures reflecting the state of the tumor immune microenvironment may also help stratify patients, though the clinical availability of such assays is limited at present[86]. Separately, the concept of minimal residual disease - measured via circulating tumor DNA (ctDNA) - has shown promise in selecting patients with MIBC for adjuvant ICI[87,88]. A ctDNA-based approach could be explored as a means of risk-stratifying mUC patients after platinum-based chemotherapy and selecting higher-risk patients for maintenance ICI.
Another consideration in the post-chemotherapy setting is when erdafitinib should be incorporated for patients with qualifying FGFR2/3 alterations and whether it should be prioritized over ICIs. FGFR alterations may be a negative ICI biomarker since they are associated with the luminal-papillary molecular subtype, which exhibits lower PD-L1 expression and decreased immune infiltration[71]. On the other hand, immunosuppressive stromal signaling appears to be reduced in FGFR3-altered tumors, and similar ICI response rates have been observed regardless of FGFR3 status in three separate cohorts[89,90]. Interestingly, in the biomarker-driven BISCAY study, the combination of durvalumab with the FGFR inhibitor AZD-4547 yielded similar response rates and outcomes as AZD-4547 alone (28.6% vs. 31.3%), suggesting a limited additional benefit from durvalumab in an FGFR-altered population[91]. The THOR phase III trial (NCT03390504) will definitively compare erdafitinib against pembrolizumab as a second-line treatment in patients with FGFR alterations.
In the front-line setting, ICI monotherapy has demonstrated activity in cisplatin-ineligible patients in early-phase trials[80,82]. However, the role of ICIs relative to carboplatin chemotherapy in this population is still evolving. For patients who are not candidates for any chemotherapy, ICI monotherapy is an appealing option. However, for those who can tolerate carboplatin chemotherapy, a maintenance ICI approach (per JAVELIN Bladder 100) may be favored over upfront ICI monotherapy, given its proven OS benefit.
PD-L1 expression is used as a biomarker among cisplatin-ineligible patients to guide the choice of upfront ICI monotherapy vs. carboplatin chemotherapy. Cisplatin-ineligible patients with PD-L1-low tumors should not be treated with upfront ICI monotherapy due to evidence of increased early mortality among PD-L1-low patients treated with upfront ICI compared to chemotherapy in the IMvigor130 and KEYNOTE-361 trials[11,12,92]. Cisplatin-ineligible patients with PD-L1-positive tumors have the option of upfront ICI or chemotherapy followed by maintenance ICI; these options have not been directly compared in clinical trials. There may be a role for other biomarkers of chemotherapy response (e.g., DDR mutations) or ICI response (discussed below) to inform the choice of upfront ICI or chemotherapy in this setting. However, as it stands, PD-L1 expression is the only ICI biomarker that has been incorporated into mUC regulatory approvals and treatment guidelines.
ICI biomarker development has lagged in therapeutic innovation driven by clinical trials. In the absence of reliable and selective ICI biomarkers, clinical trials are testing ICI-based combinations in biomarker-unselected populations. Results to date have been mixed for the combination of chemotherapy with ICI (chemo-ICI) and negative for dual ICI-ICI therapy. Pembrolizumab with platinum-based chemotherapy did not improve PFS or OS over chemotherapy alone in KEYNOTE-361[12]. Conversely, PFS was improved by combining atezolizumab with platinum-based chemotherapy in IMvigor130; OS results are still pending[11]. The ICI-ICI combination of durvalumab (anti-PD-L1) and tremelimumab (anti-CTLA-4) failed to improve OS compared to platinum chemotherapy in the DANUBE trial[13].
Moving forward, we can expect results from trials testing ICI-ICI combinations with or without chemotherapy and trials testing chemotherapy-free combinations of ICIs and targeted therapies [Figure 2]. Examples of the former include the NILE (NCT03682068) and CheckMate 901 (NCT03036098) trials. NILE is comparing durvalumab plus chemotherapy, durvalumab plus tremelimumab plus chemotherapy, and chemotherapy alone in the upfront mUC setting. Due to the negative results in a biomarker-unselected population in DANUBE, the primary endpoint of NILE has been revised to focus on patients with PD-L1-expressing tumors[93]. As for chemotherapy-free regimens, the most promising at present is the combination of EV/P, which has produced overall response rates of 73.3% in the first-line cisplatin-ineligible setting and has been granted FDA breakthrough designation[14]. The combination will be tested against upfront chemotherapy in phase III EV-302 trial (NCT04223856) and is being tested in additional settings in the multi-cohort EV-103 study (NCT03288545).
Figure 2. Potential landscape. Potential metastatic urothelial carcinoma (mUC) treatment landscape. Clinical factors which influence treatment selection, such as cisplatin-eligibility, are highlighted in green. Biomarkers are highlighted in yellow. Current FDA-approved treatments are in blue while investigational treatment options are in purple. The combination of chemotherapy and atezolizumab (Chemo-ICI) showed PFS benefits in the IMvigor130 (NCT02807636) trial but OS results are still pending. The combination of enfortumab vedotin with pembrolizumab (EV/P) is being tested in various settings, including first-line against platinum chemotherapy (EV-302, NCT04223856), first-line cisplatin-ineligible (EV-103, NCT03288545), and second-line (EV-103; EV-201, NCT03219333). Other combinations and targeted therapies are under investigation. PD-L1: Programmed death-ligand 1; ICI: immune checkpoint inhibitor; 1L: first-line; 2L: second-line; 3L: third-line.
While synergistic combinations are possible, it has been argued that drugs in combination regimens often act independently[85]. That is, the benefit any given patient derives from a combination of A and B is due entirely to either A or B. On a population level, combination regimens improve outcomes by pooling together A-responders and B-responders. In this model, the combination of A and B is effectively a workaround for the lack of a biomarker, for if a biomarker were available, A-responders and B-responders would be treated with their most effective single agent. In practice, there may be other benefits from independently acting combinations, e.g., patients with progression may deteriorate clinically and become unable to tolerate an effective next line of therapy. Nevertheless, there is a clear need for better ICI biomarkers to guide the integration of ICIs into each patient’s treatment course.
PD-L1
Meta-analyses of prospective trials show that, overall, PD-L1 expression is associated with radiographic response to ICIs in mUC patients[94,95]. However, even among PD-L1 positive patients, single-agent ICI response rates are low and variable across randomized trials, ranging from 20%-40%[3,11-13,96].
Several technical and biological factors may limit the performance of PD-L1 expression as a biomarker. PD-L1 expression is measured through IHC staining. However, IHC assays differ in both the antibody used and in how the resulting stains are scored. Scoring systems differ on whether PD-L1 staining is scored on tumor cells, immune cells, or both, and on the threshold value for PD-L1 positivity. Furthermore, nearly every anti-PD-1/PD-L1 agent has been co-developed with its own assay, making it difficult to compare “PD-L1 positive” subgroups across trials. Efforts to compare PD-L1 assays in non-small cell lung cancer have found consistent staining across some assays, but not others[97,98], and have described limited inter-rater reliability when scoring PD-L1 staining on immune cells[99].
In addition to discrepancies between PD-L1 assays, there may also be heterogeneity of PD-L1 expression within tumors and between primary and metastatic sites, which is not captured by limited biopsies. Clonal evolution of tumors over time and over the course of treatment is also impractical to monitor using invasive biopsy procedures. Emerging non-invasive technologies, such as liquid biopsy and immune-targeting tracers for positron emission tomography (ImmunoPET)[100-102], may circumvent some of these limitations and pave the way for more comprehensive and longitudinal monitoring of PD-L1 or other ICI biomarkers.
Mutation burden
Bladder cancers are among the most highly mutated cancers[103]. Nonsynonymous tumor mutations can lead to the formation of novel peptide sequences, or neoantigens, which the immune system recognizes as foreign and serve as the targets for immune-mediated tumor cell killing. TMB has been linked to ICI response across cancer types and in mUC specifically[79,80,104-106].
In 2020, pembrolizumab received accelerated approval from the FDA for any solid tumor with TMB ≥ 10 mutations/Mb without satisfactory treatment alternatives[107]. This indication is less relevant to mUC since ICIs are already routinely integrated into the treatment plan. However, TMB may be useful as an adjunct to other ICI biomarkers. Notably, TMB is not correlated with PD-L1 expression[108]. Exploratory analyses of prospective trials in mUC suggest that the combination of TMB and PD-L1 may more effectively distinguish ICI responders and non-responders than either biomarker alone[109,110].
Challenges in implementing TMB as a biomarker include selecting an optimal cutoff and harmonizing assays. Although the pembrolizumab approval used a uniform cutoff of 10 mutations/Mb across all tumors, tumor-specific cutoffs may be more appropriate[104,111]. Efforts have also been made to harmonize TMB measurements, which may vary depending on the size and composition of the panel used for sequencing[112].
In addition to the quantity of mutations, there may also be qualitative aspects of mutations that contribute to immunogenicity. For example, mutation clonality may matter - whether the mutation is present in all cancer cells (clonal) or only a subset of cells (subclonal)[95]. Short insertion-deletion (indel) mutations may be more likely to generate frameshift mutations which create a greater quantity of immunogenic neoantigens[113]. Neoantigens and their interaction with the patient’s HLA alleles may also play a role[95,105,114]. Mutational signatures, which reflect underlying mutagenic processes at work in tumors, have also been linked to ICI response. Mutational signatures attributed to the APOBEC family of cytidine deaminases are common in bladder cancers[36,103] and have been linked with favorable ICI response in prospective mUC trials[95,105,110].
According to a recent meta-analysis by Litchfield et al.[95], which combined data from over 1000 ICI-treated patients (including 387 mUC patients) and identified clonal TMB as the strongest predictor of response overall, followed by total TMB, they found that clonal TMB and the APOBEC signature were among the most important features associated with response in a multivariable model predicting ICI response in bladder cancer.
Somatic alterations
Mutations and copy number alterations (CNAs) affecting specific genes have been linked to ICI outcomes. In their meta-analysis, Litchfield et al.[95] identified TRAF2 loss as a predictor of ICI response and CCND1 amplification as a marker of ICI resistance both across cancer types and in UC specifically. Separately, biomarker analyses from clinical trials have found that somatic mutations in DDR or cycle regulator genes are associated with both TMB and response[105,106].
TRAF2 is a tumor necrosis factor (TNF) receptor-associated protein which inhibits TNF-mediated apoptosis[115]. Interestingly, TRAF2 loss had been independently identified in a CRISPR/Cas9 screen for genomic alterations sensitizing tumors to T cell killing[116]. In the Litchfield et al.[95] meta-analysis, loss of the 9q34 cytoband, and specifically the TRAF2 locus within it, was the most significant CNA associated with ICI response (lost in 44% of responders vs. 30% of non-responders, P = 6.9 × 10-5).
CCND1 encodes Cyclin D1, a cell cycle regulator that has also been shown to negatively regulate PD-L1 protein levels[117]. In the Litchfield meta-analysis, UC was the cancer type with the most CCND1 amplification. Using various UC and pan-cancer clinical cohorts, they showed that higher CCND1 expression levels were associated with lack of ICI response, and CCND1 amplification was associated with worse OS among ICI-treated patients but was not associated with OS among non-ICI treated patients, suggesting a predictive relationship. However, they were unable to test for a formal interaction due to differences in the ICI and non-ICI treated cohorts[95].
Mutations in DDR pathway genes were associated with improved outcomes in exploratory analyses of both the IMvigor210 and JAVELIN Bladder 100 trials. However, in the IMvigor210 analysis, Mariathasan et al.[105] found that DDR mutations were not predictive of outcomes independent of TMB. This may also be the case in the JAVELIN study, but the biomarker analyses are currently only available in abstract form[86].
Gene expression
In contrast to somatic alterations in DNA, which are essentially fixed for each cell, gene expression is dynamic and reflects ongoing cellular processes at sampling. Gene expression profiling might therefore capture the state of the tumor and its microenvironment in a way that DNA sequencing does not. Several studies have used samples from mUC clinical trials to identify gene expression signatures correlated with response or resistance to ICI therapy[81,86,105,118].
Two broad categories of gene expression signatures have been linked to ICI response in prospective mUC cohorts: a group of genes reflecting cytotoxic T cell activity and a group of genes reflecting immunosuppressive stromal signaling. The former has been associated with ICI response, while the latter has been associated with ICI resistance. These signatures remain exploratory pending validation in additional prospective cohorts.
Gene expression analyses of data from TCGA, the CheckMate-275 trial of nivolumab, and the IMvigor210 trial of atezolizumab converged on the observation that certain types of stromal cell signaling contribute to an “immune-excluded” phenotype, wherein CD8 T cells are separated from tumor cells by dense connective tissue[105,119]. Notably, TGFβ signaling can promote the differentiation of mesenchymal precursors into fibroblasts[120-122], and in IMvigor210 both a TGFβ ligand (TGFB1) and receptor (TGFBR2) were associated with non-response and reduced OS[105]. Hypothesizing that TGFβ signaling in fibroblasts might be a biomarker for ICI response, Mariathasan et al.[105] showed that a pan-fibroblast TGFβ response signature (F-TBRS) was associated with response in immune-excluded tumors. They further showed in murine tumor models that anti-TGFβ antibodies enhanced the efficacy of anti-PD-L1 therapy. A higher F-TBRS signature was also associated with worse OS for patients treated with atezolizumab rather than platinum-chemotherapy in the IMvigor130 trial[110].
A variety of inflammatory gene signatures reflecting CD8 T cell activity and/or interferon-gamma signaling have been associated with ICI response in mUC[81,86,105,118]. Some recurrent genes in these signatures include: CCL5, CD27, CD8A, CXCL9, CXCL10, CXCR6, GZMA, GZMB, IDO1, IFNG, LAG3, PRF1, STAT1, and TBX21. A 25-gene interferon-gamma signature was associated with response in the CheckMate-275 trial of nivolumab[81]. An 8-gene subset of that signature focused on CD8 T effector activity was positively associated with response in IMvigor210[105]. CXCL9 and CXCL10 expression were individually associated with ICI response in both IMvigor210 and CheckMate-275[81,105]. Notably, CXCL9 expression was one of the strongest predictors of ICI response in the Litchfield et al.[95] meta-analysis of ICI biomarkers across tumor types.
Molecular subtypes
The TCGA molecular classification has been the most used schema in ICI clinical trials. The classification scheme, comprising 4 subtypes, was developed in 2014 using gene expression data from 131 muscle-invasive bladder tumors[65]. It was updated in 2017 using an expanded cohort of 412 muscle-invasive specimens, with a fifth “neuronal” subtype added[36], though the analyses in mUC ICI trials have focused on the initial 2014 classification or have omitted the neuronal subtype.
Associations between molecular subtypes and ICI responses have been inconsistent across ICI trials and agents. Data from the IMvigor210 trial of atezolizumab found the highest response rates among the luminal II subtype[79,80,105], whereas the CheckMate-275 trial of nivolumab found the highest response among the basal I subtype[81]. The JAVELIN Bladder 100 trial of maintenance avelumab found no association between subtypes and OS[123].
It may be that the molecular factors associated with ICI response do not segregate neatly into existing molecular classifications. In the analysis of IMvigor210, Mariathasan et al.[105] found that combining a separate molecular classification scheme (the Lund scheme) with the TCGA scheme could improve the separation of responders and non-responder. In this analysis, they found that about half of tumors classified as luminal II in the TCGA scheme could also be classified as genomically unstable (GU) according to the Lund scheme. Tumors that were both GU and luminal II had high TMB and low expression of the F-TBRS score associated with fibroblast TGFβ activity. On the other hand, tumors that were luminal II but not GU had low TMB, a high F-TBRS score, and had a significantly lower overall response rate[105].
Tumor microenvironment factors
Immune checkpoint inhibitors are not directly cytotoxic but rather alter immune cell signaling to promote immune-mediated destruction of tumor cells. Given this indirect mode of action, the factors that influence ICI outcomes extend beyond the tumor cells and include interactions between tumor, immune, and stromal cells within the tumor microenvironment (TME)[124,125]. Thus far, we have discussed examples of both tumor-intrinsic (mutation burden, somatic alterations) and tumor-extrinsic (immune cell infiltration, fibroblast activity) candidate ICI biomarkers. Additional tumor-extrinsic ICI biomarkers may emerge as our understanding of the TME matures.
Early efforts to examine the TME in relation to ICI response used immunohistochemistry to correlate the spatial arrangement of tumor-infiltrating lymphocytes with outcomes[126]. This gave rise to the paradigm of immune inflamed, immune cold, and immune excluded phenotypes, with immune inflamed tumors generally having better responses[105,126]. Deconvolution algorithms have also been used to infer the immune composition of the TME from bulk tumor RNA[127,128]; these inferred cell populations can then be correlated with ICI outcomes[129]. More recently, tools such as single-cell RNA-sequencing (scRNA-seq) and multiplex immunohistochemistry, are enabling higher-resolution analysis of the TME and its relationship to ICI response across cancer types[130,131]. In contrast to bulk gene expression profiles, scRNA-seq enables the direct quantification and characterization of distinct cellular subpopulations. In urothelial carcinoma, this has been used to elucidate the heterogeneity of tumor, immune, and stromal cells[132-134].
Fibroblasts are of particular interest in mUC given the identification of a fibroblast-associated TGFβ signature as a predictor of atezolizumab response in the IMvigor210 analysis[105]. Note that this analysis did not use scRNA-seq - the TGFβ signature was developed in vitro by exposing fibroblasts to TGFβ ligands and measuring changes in gene expression. The signature was then applied to bulk RNA-seq data as a proxy for TGFβ activity in fibroblasts. A separate single-cell analysis of 8 bladder cancer samples by Chen et al.[133] identified a subset of fibroblasts that were a major source of pro-proliferation growth factors and CXCL12 - a chemokine associated with the accumulation of immunosuppressive tumor-associated macrophages[133,135]. However, TGFβ signaling in these fibroblasts was not reported.
Single-cell profiling may reveal novel determinants of ICI response which can be translated into biomarkers. For example, in a single-cell analysis of 7 bladder tumors, Oh et al.[132] identified cytotoxic CD4+ T cell populations which had not been previously recognized in bladder cancer. A gene expression signature derived from these novel CD4 T cells was applied to bulk RNA sequencing data from the IMvigor210 study and was predictive of atezolizumab response among patients with an inflamed tumor microenvironment.
In another example combining bulk and single-cell analyses to dissect the UC TME, bulk gene expression data from two clinical trials were first used to identify two signatures associated with ICI response and resistance[136]. The authors sought to identify the cell types responsible for each signature using single-cell data. Unexpectedly, they found that many cell types - and myeloid cells in particular - expressed a mix of genes from both signatures rather than being restricted to one signature. This led them to define a score based on the ratio of the two signatures within myeloid cells (Msc21R). Intriguingly, this single-cell expression-based score was associated with ICI outcomes when measured in the pre-treatment peripheral blood monocytes of 10 mUC patients.
While scRNA-seq remains limited to research settings for now, single-cell insights from the TME are likely to influence biomarker development moving forward.
TARGETED THERAPIES BIOMARKERS
Molecularly targeted therapies have also evolved over recent years, expanding the landscape of cancer treatment options available to patients. The target is often a mutated or overexpressed molecule with a critical role in tumor progression while being less important for normal cell function, creating a therapeutic window that can be exploited. In UC, current FDA-approved targeted therapies are the FGFR inhibitor erdafitinib, the Nectin-4 ADC EV, and the Trop-2 ADC SG.
Erdafitinib, a pan-FGFR-kinase inhibitor, was granted accelerated approval in 2019 for patients with locally advanced or metastatic UC with progression after platinum-based chemotherapy and known susceptible FGFR2/3 alterations. The specific alterations include FGFR3 mutations (R248C, S249C, G370C, Y373C) or FGFR2/3 gene fusions (FGFR2-BICC1, FGFR2-CASP7, FGFR3-TACC3, FGFR3-BAIAP2L1). Accelerated approval was based on a phase II trial demonstrating overall response rates of 30%-40% in this biomarker-driven population[7,137]. The confirmatory phase III THOR trial (NCT03390504) is currently underway, directly comparing erdafitinib to pembrolizumab or single-agent chemotherapy in previously treated mUC.
EV is an ADC consisting of a monoclonal antibody specific for Nectin-4 conjugated to monomethyl auristatin E (MMAE), a microtubule-disrupting agent[138,139]. Accelerated approval was granted for locally advanced or metastatic UC after prior platinum-based chemotherapy and ICI as a result of phase II EV-201 trial. In the confirmatory phase III EV-301 trial, EV conferred a significant survival benefit over standard chemotherapy in the post-chemo/post-ICI setting, leading to regular FDA approval[4]. Notably, EV has shown benefit and is approved for treatment without regard to Nectin-4 levels.
The third targeted therapy approved in mUC is SG, a monoclonal antibody specific for Trop-2 conjugated with SN-38, the active metabolite of irinotecan[140]. Accelerated approval for SG was recently granted in April 2021 after the phase II TROPHY-U-01 trial demonstrated a 27% ORR and 10.9 months median OS in the post-chemo/post-ICI setting[5]. Similar to EV, SG has been tested and approved without regard to the levels of its target, Trop-2.
Several other targets are under investigation in UC. Below we describe approved and prospective targeted therapies in more detail, with an emphasis on biomarkers that may guide treatment options.
FGFR
The FGFR proteins comprise a family of four transmembrane receptor tyrosine kinases, FGFR 1-4, with roles in cellular proliferation, differentiation, apoptosis, migration, and angiogenesis[141,142]. Upon binding to their ligand, these receptors undergo dimerization and autophosphorylation resulting in subsequent activation of the RAS-MAPK and PI3K signaling pathways. Dysregulation of the FGFR pathway has been linked to oncogenesis in multiple cancer types, including UC[143]. FGFR3 alterations are observed in approximately 81% of non-invasive UC and up to 54% of invasive UC[144], and are more commonly seen in the luminal-papillary molecular subtype that comprises 35% of MIBC[36]. The most common FGFR3 mutation is S249C (61% of FGFR3-mutated UC), followed by Y375 (19%), R248C (8%), and G370C (6%), all conferring downstream signaling activation through ligand-independent receptor dimerization or constitutive receptor activity[145]. Amplification of the FGFR genes occurs in UC with a prevalence of approximately 7% for FGFR1 and 2% for FGFR3[146]. Finally, FGFR fusion pairings also lead to signaling activation, most notable between FGFR3 and fusion partner TACC3 in approximately 2.6% of UC[65,147].
As described above, erdafitinib has been approved for use in previously treated mUC with susceptible FGF2/3 alterations. It remains to be seen whether erdafitinib may benefit patients in combination with other therapies or in earlier lines of therapy. The phase Ib/II NORSE trial (NCT03473743) may answer some of these questions, evaluating erdafitinib in combination with cetrelimab (anti-PD-1) and platinum chemotherapy in FGFR-altered patients with or without prior treatment and in treatment-naïve cisplatin-ineligible patients[148].
Multiple additional FGFR inhibitors have also been evaluated in UC. Here we highlight notable results and differences between them. Infigratinib (BGJ398), an FGFR1-3 inhibitor, was tested in patients with the same FGFR alterations as erdafitinib, as well as Y375C, K652M/T, K652E/Q[149], and is currently being studied in phase III PROOF302 trial (NCT04197986) as adjuvant therapy for cisplatin-ineligible bladder cancer or upper tract UC.
Vofatamab (B-701), a monoclonal antibody that inhibits FGFR3, is being tested in FGFR3-altered mUC in the post-chemotherapy setting in the phase Ib/II FIERCE-21 trial[150,151]. Of note, the phase Ib/II FIERCE-22 trial found promising response rates for vofatamab in combination with pembrolizumab in both FGFR3-mutant (ORR 43%) and FGFR3-wild-type (ORR 33%) patients[152]. Vofatamab is known to inhibit wild-type FGFR3, which may play a part in reversing the ICI-insensitivity attributed to FGFR signaling[152].
Derazantinib (ARQ087) is an FGFR2 selective inhibitor in the phase Ib/II FIDES-02 trial as monotherapy or in combination with atezolizumab for cisplatin-ineligible advanced UC with FGFR1-3 mutations and fusions. The inhibitor also has known immunomodulatory activity on macrophages via CSF1R[153,154].
Additional FGFR inhibitors include Debio 1347, an FGFR1-3 inhibitor being tested only for FGFR1-3 fusions[155], TAS-120, an FGFR1-4 inhibitor under evaluation for FGFR alterations that include amplifications[156], pemigatinib, an FGFR1-2 inhibitor being studied for NMIBC with recurrent low or intermediate risk disease, and, dovitinib, an FGFR1-3 inhibitor which demonstrated limited efficacy[157].
FGFR1-3 mRNA overexpression has also been tested as a biomarker, with equivocal results. Among patients with increased FGFR1/3 mRNA levels, rogaratinib demonstrated similar outcomes as chemotherapy in the post-platinum setting in phase II/III FORT-1 trial. However, the response rate was highest among those with FGFR3 somatic alterations, suggesting that mRNA overexpression may be a less selective biomarker than bona fide somatic alterations[158]. Conversely, the phase Ib/II FORT-2 trial reported favorable responses for rogaratinib in combination with atezolizumab among FGFR1-3 mRNA overexpressing mUC patients regardless of FGFR DNA alterations or PD-L1 level, though the sample size was limited in this early-phase trial[159]. The utility of FGFR mRNA overexpression remains unclear, especially in the setting of FGFR inhibitor monotherapy.
Finally, ctDNA may serve as both a prognostic and treatment response biomarker for FGFR inhibitors. In the BISCAY trial, which tested durvalumab in combination with targeted therapies in biomarker-selected populations, FGFR alterations identified in tissue samples could be accurately detected in ctDNA. Higher baseline ctDNA levels correlated with worse OS. Furthermore, FGFR alterations in ctDNA declined with response to therapy and increased with clinical progression[91].
It is also important to pinpoint markers of treatment resistance, either at baseline or acquired after therapy, that may aid in clinical decision making. There are multiple gatekeeper mutations located in the FGFR ATP-binding pocket known to confer resistance to FGFR inhibitors[160]. In particular, infigratinib has a 5-10-fold decreased efficacy with the K650E gatekeeper mutation[161]. Additionally, there are multiple secondary FGFR2 mutations are identified as sources of resistance, such as V564F after infigratinib treatment[162]. While these tumor cells appear to maintain dependence on the FGFR signaling pathway, activation of other pathways such as PI3K-AKT and RAS-MAPK also serve as mechanisms of resistance[160,163,164]. Lysosome-mediated TKI sequestration, activating gene fusions, and epithelial-mesenchymal transition are further mechanisms that limit FGFR inhibitor efficacy[160,165]. These markers may serve to predict non-responders to FGFR inhibition, identify specific inhibitors that may be more efficacious for an individual, or guide discontinuation of FGFR-targeted therapy.
Nectin-4
Enfortumab vedotin is an ADC targeting Nectin-4, a transmembrane protein involved in cellular adhesion and upregulated in UC with limited expression in benign tissue[166]. Despite the targeted nature of EV, Nectin-4 is not currently utilized as a biomarker in patient selection. The EV-101 phase I trial initially required Nectin-4 expression for inclusion, but the protocol was later amended to remove that requirement because nearly all screened tumors expressed Nectin-4 at high levels[167]. Subsequently, the pivotal phase III EV-301 trial demonstrated an OS benefit for EV in a biomarker-unselected population[4].
Nevertheless, emerging treatment options in the same clinical setting (e.g., sacituzumab govitecan) provide an impetus to identify EV biomarkers for treatment selection and prioritization. In a recent abstract, NECTIN4 gene expression was found to vary by molecular subtype and was enriched in luminal-type UC. In both luminal and basal bladder cancer cell lines, overexpressing and knocking out NECTIN4 conferred sensitivity and resistance, respectively, to EV in vitro[168]. Nectin-4 protein levels vary by disease stage (87% NMIBC vs. 58% MIBC) and by morphology (e.g., 28% in micropapillary tumors, 63% in plasmacytoid)[169]. It remains to be seen whether Nectin-4 expression at the RNA or protein level is associated with clinical outcomes or may interact with molecular subtype or variant histology.
Ongoing trials with EV include the phase Ib/II EV-103/KEYNOTE-869 trial (NCT03288545) evaluating the ADC in combination with pembrolizumab and/or chemotherapy for MIBC, locally-advanced, and metastatic UC[170], with preliminary analysis showing promising results in combination with pembrolizumab as first-line therapy for cisplatin-ineligible patients[171]. EV-302 (NCT04223856) is evaluating EV with pembrolizumab compared to standard of care chemotherapy for untreated locally advanced or metastatic UC, NCT04878029 is looking at EV with cabozantinib for advanced UC, and NCT04724018 is testing EV with SG post-chemo and ICI, all of which will not be biomarker-driven.
Trop-2
Trop-2 is a transmembrane glycoprotein involved in calcium signaling and cellular proliferation, with reported overexpression in up to 83% of UC, and, importantly, limited expression in normal tissues[172,173]. High Trop-2 expression has also been associated with more advanced disease and recurrence[174]. Similar to EV, SG was studied and approved in a biomarker-independent manner.
As a biomarker, Trop-2 overexpression in other malignancies has been paradoxically linked to growth promotion after oxaliplatin[140], and is associated with cisplatin resistance[175] and response to AKT inhibitors[176]. In breast cancer, Trop-2 expression is differentially expressed among hormone receptor-positive and triple-negative disease[177], and biomarker analysis of a phase III trial demonstrated that while SG benefitted patients regardless of Trop-2 expression, the benefit was greatest among the high Trop-2 expression subgroup[178]. As more options become available in the post-chemo/post-ICI setting, biomarker selection will become increasingly important, and more intricate analysis of mRNA expression and molecular subtypes may also prove meaningful for Trop-2.
Ongoing SG trials include the phase III TROPiCS-04 trial evaluating SG for advanced UC progressing after platinum-based chemotherapy and ICI[179], and the phase Ib/II umbrella trial with SG in combination with atezolizumab for cisplatin-ineligible MIBC and platinum-refractory advanced UC[180].
ErbB (Her2, EGFR)
Human epidermal growth factor receptor 2 (HER2/ErbB2) is a cancer biomarker with approved targeted therapies for positive breast and gastroesophageal cancer patients. The protein is one of four in a family of receptors with tyrosine kinase activity involved in cellular proliferation and differentiation[181], with overexpression prevalence in UC cohorts as high as 76%[182,183].
Multiple Her-2 inhibitors have been tested in UC, with limited success. Trastuzumab initially showed promising results for advanced chemo-naive UC in combination with platinum-based chemotherapy[184], but a subsequent trial showed no added benefit[185]. Lapatinib, a Her-2 and EGFR pathway inhibitor, also failed to improve outcomes in a phase III trial for HER1/2-positive mUC after first-line chemotherapy[186].
Conversely, afatinib, a Her-2/4 and EGFR inhibitor, has demonstrated biomarker-driven potential for platinum-refractory mUC. PFS at three months was 83% in patients with HER2, EGFR, ERBB3, or ERBB4 alterations, compared to 0% of patients without these alterations[187]. The follow-up phase II LUX-Bladder 1 trial assessing afatinib for ERBB1-3 altered mUC in the post-platinum setting is ongoing[188]. Additionally, the basket trials My Pathway (NCT02091141)[189] and NCI-MATCH (NCT02465060) are currently evaluating trastuzumab and pertuzumab in bladder cancer with HER2 overexpression or amplification.
Multiple ADCs targeting Her-2 are also being tested in UC. Disitamab vedotin (RC48-ADC), a Her-2 antibody conjugated with MMAE, demonstrated promising responses for advanced UC after at least 1 prior line of therapy[190]. Trastuzumab deruxtecan (DS-8201), an ADC pairing trastuzumab with a topoisomerase I inhibitor, is in an ongoing phase Ib trial (NCT03523572) in combination with nivolumab for Her-2 positive mUC after progression on platinum-based chemotherapy, and the basket trial NCT02675829 is testing ado-trastuzumab emtansine (TDM-1) in a HER2-amplified bladder cancer cohort[191].
Further trial data may elucidate the optimal utilization of Her-2 as a biomarker for either Her-2 inhibitors or ADCs. Gene expression, gene amplification, and protein level by IHC, FISH, or serum levels may all play a role in treatment and patient selection. Molecular subtypes may also be useful in guiding EGFR-based therapy, as basal-like tumors demonstrated increased EGFR pathway activity and sensitivity to EGFR-based treatment in pre-clinical models[69].
PI3K/AKT/mTOR, MAPK pathway
The PI3K/AKT/mTOR pathway serves to promote cellular growth, proliferation, and angiogenesis, and regulates apoptosis and autophagy. PI3K is a lipid kinase activated via phosphorylation by RTKs and GPCRs upon ligand binding. The protein then phosphorylates and converts PIP2 into PIP3, a process inhibited via dephosphorylation by the tumor suppressor PTEN. PIP3 subsequently recruits the kinase AKT, which functions to inhibit apoptosis through Bcl-2 and activates mTOR through suppression of TSC1 and TSC2[192]. Genomic alterations throughout this pathway result in increased downstream activity and are common in many malignancies. Aberrations in the pathway are seen in up to 72% of UC[193], commonly through PIK3CA mutations (21%-25% of MIBC)[65,194,195], loss of PTEN expression (39%-94%)[195-199], loss of heterozygosity of TSC1 (40%-50%) or TSC2 (15%)[195,200], or due to aberrations in the upstream FGFR or ERBB pathways as outlined above.
Everolimus, an mTOR inhibitor, has been tested in the post-chemotherapy setting for UC[201]. Notably, one patient with an exceptional response was found to have a loss-of-function mutation in TSC1, generating significant interest in mTOR pathway mutations as biomarkers for everolimus response. When everolimus was tested in combination with the TKI pazopanib, TSC1, TSC2, or mTOR mutations were identified in the only complete responder (CR), and two of the three partial responders[202], and subsequent analysis of the CR revealed two distinct activating mTOR mutations, both validated in vitro and conferring sensitivity to mTOR inhibition[203]. However, follow-up studies have failed to yield clinically relevant efficacy for mTOR inhibition in UC treatment, including additional inhibitors such as sapanisertib, which showed minimal response for TSC1/2-mutated mUC patients[204].
The PI3K inhibitor, buparlisib (BKM-120), was also evaluated in the post-platinum setting, and, similarly, analysis of multiple responders revealed TSC1 or PIK3CA mutations. However, an expansion cohort for patients with PI3K pathway alterations was terminated after no patients achieved disease control[205]. Copanlisib and GSK2636771 are additional PI3K inhibitors currently under investigation in the basket NCI-MATCH trial (NCT02465060) for patients with PI3KCA-activating mutations or loss/mutations of PTEN[206].
AKT inhibitors (MK-2206, AZ7328) have also been tested in pre-clinical studies, with sensitivity dependent on PIK3CA mutations and resistance seen with PTEN, TSC1, and RAS alterations[207,208]. Further, dual PI3K and mTOR inhibitors, such as dactolisib (BEZ235) and omipalisib (GSK2126458), have also undergone evaluation for UC[209,210].
PARP/DNA repair
PARP genes can detect DNA damage and subsequently recruit downstream proteins involved in DNA repair. The PARP inhibitors olaparib, rucaparib, niraparib, and talazoparib have gained FDA approvals, primarily as monotherapy for ovarian, breast, prostate, and pancreatic cancer with germline or somatic BRCA1/2 mutations, owing to the synergistic synthetic lethality in tumors with HRR deficits[211]. BRCA1/2 mutations occur in approximately 1.5% and 1.4%, respectively, of UC[49].
Rucaparib was tested in UC in the phase II ATLAS trial, with no observed relationship between HRR deficiency and efficacy; only 4 of the 97 patients had BRCA1/2 deficiencies[212]. Ongoing phase II trials are evaluating olaparib in mUC with at least one somatic DDR gene alteration (NCT03448718, NCT03375307). Olaparib did not appear to confer additional benefit when combined with durvalumab in the phase Ib BISCAY study (NCT02546661), even among those with HRR mutations[91]. The combination is also being evaluated for platinum-ineligible mUC in phase II BAYOU trial (NCT03459846).
It remains to be seen whether PARP inhibition in HRR-deficient patients will prove efficacious for UC. However, as outlined above, various DNA repair genes have potential as predictive biomarkers for chemotherapy response, and chemotherapy itself is also involved in DNA repair dysregulation, raising the possibility of synergistic efficacy with PARP inhibition.
Additional TKIs/VEGF inhibitors
Multiple non-specific TKIs have been evaluated for UC. Sorafenib, a multi-targeted TKI acting on the VEGF, RAF/MEK/ERK, and PDGF pathways, was tested in the neoadjuvant setting in combination with cisplatin/gemcitabine. Responders demonstrated higher rates of mutations in DNA-repair genes, RAS-RAF pathway genes, chromatin-remodeling genes, and HER-family genes (of note only HER-family genes were statistically significant given the study’s limited power, 36.4% vs. 0%)[24]. The TKI is also under evaluation for post-platinum mUC.
Cabozantinib, which has immunomodulatory properties involving Tregs and myeloid-derived suppressor cells, has also been tested in platinum-refractory mUC. Biomarker analyses have suggested that cabozantinib may counteract an immunosuppressive TME, providing the rationale for its combination with ICIs[213]. A combinatorial phase I trial testing cabozantinib with nivolumab and/or ipilimumab has shown promising results, with baseline circulating tumor cell subsets (defined by EpCAM, MET, and CXCR4 expression) associated with median OS[214]. Ongoing trials such as ARCADIA (NCT03824691) and COSMIC-021 (NCT03170960) are testing cabozantinib-ICI combinations with plans for extensive correlative studies to support biomarker development, including immunophenotyping, gene expression profiling, and somatic mutation testing in both tumor and plasma[215,216].
Other recently reported trials of targeted therapies with the potential for biomarker analysis include the RANGE study of ramucirumab with docetaxel (NCT02426125), and the multi-cohort study of sitravatinib in mUC (NCT03606174). The RANGE study found that ramucirumab (a monoclonal antibody targeting VEGFR-2) with docetaxel improved PFS but not OS among previously treated mUC patients[217]. However, high PD-L1 expression was associated with improved PFS, and further biomarker analyses may uncover patient subsets who derive more benefit. Sitravatinib, another multi-targeted TKI with immunomodulatory activity, is being tested in combination with nivolumab, and biomarker analyses are planned[218].
Multi-targeted TKIs are an intriguing future prospect for mUC management. Many have immunomodulatory properties, which make them promising candidates for ICI combinations. However, their pleiotropic effects through multiple pathways may mask the patient subsets who derive the most benefit, making biomarker analyses even more essential.
CONCLUSION
There are now three distinct therapeutic classes for the treatment of metastatic urothelial cancer: chemotherapy, immunotherapy, and targeted therapies [Figure 3]. This presents medical oncologists with the challenge of finding the optimal sequence and combination of these agents to maximize outcomes for their patients. The precision medicine paradigm aims to divide urothelial cancer into increasingly specific subtypes, each with its own optimal treatment plan. However, realizing this vision requires both a variety of effective treatment options and reliable biomarkers to distinguish the relevant disease states. As it stands, biomarker development has lagged in therapeutic development, with only PD-L1 expression and FGFR alterations playing a routine role in treatment selection.
Figure 3. Treatment Classes. Summary of biomarkers for metastatic urothelial carcinoma by therapeutic classes.
Nevertheless, there is reason to be hopeful that greater resolution of biomarker-defined subgroups will be achieved. Research into the basic mechanisms underlying urothelial cancer and its response to therapy has already yielded various candidate biomarkers, as detailed in this review. More candidate biomarkers will be identified as researchers use increasingly precise and comprehensive molecular assays to interrogate tumor biology, such as single-cell RNA sequencing and untargeted proteomics. Clinical molecular assays are also becoming more advanced. Sequencing panels are including more genes and may ultimately include the whole exome[219]. Commercial transcriptomic tests are being introduced into the clinic[220]. Notably, circulating tumor DNA is routinely sequenced in other cancers and has shown promise as a biomarker for both treatment response and prognosis in mUC. These practical advances, as much as basic and translational developments, are essential to realizing precision medicine in the clinic.
However, there are also reasons to be circumspect. Tumor biology is complex, and there may be a limit to the resolution that can be achieved by biomarkers based on a single marker or on a single type of biological data. However, more complex biomarkers may be challenging to deploy clinically. Certain investigative biomarkers utilize novel methods (e.g., single-cell sequencing) that may not be accessible to clinicians, though it is sometimes possible to derive related biomarkers using clinically available methods. Finally, biomarker discovery and validation require large amounts of curated data, which in turn requires significant coordination and foresight to accrue. Several databases and consortia have attempted to meet this challenge, but in general, these public efforts have not reached the necessary scale. More work and resources are needed to accelerate biomarker discovery, development, and translation.
In conclusion, the accelerating progress in therapies for metastatic urothelial cancer necessitates greater investment in biomarker discovery and validation to enable optimal treatment for bladder cancer patients.
DECLARATIONS
AcknowledgementThe authors gratefully acknowledge Jill Gregory for her medical illustrations.
Authors’ contributionsDrafting of the manuscript: Jun T, Anker J
Review, editing, and supervision: Galsky MD
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestTomi Jun is employed by Sema4. Matthew Galsky has received research funding from Bristol Myers Squibb, Novartis, Dendreon, Astra Zeneca, Merck, Genentech; has consultant or advisory board roles for Bristol Myers Squibb, Merck, Genentech, AstraZeneca, Pfizer, EMD Serono, Seattle Genetics, Janssen, Numab, Dragonfly, GlaxoSmithKline, Basilea, UroGen, and Rappta Therapeutics.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Bellmunt J, Théodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009;27:4454-61.
2. Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 2020;383:1218-30.
3. Bellmunt J, de Wit R, Vaughn DJ, et al. KEYNOTE-045 Investigators. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015-26.
4. Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med 2021;384:1125-35.
5. Tagawa ST, Balar AV, Petrylak DP, et al. TROPHY-U-01: a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol 2021;39:2474-85.
6. Loriot Y, Balar A, Petrylak D, et al. LBA24 TROPHY-U-01 cohort 1 final results: a phase II study of sacituzumab govitecan (SG) in metastatic urothelial cancer (mUC) that has progressed after platinum (PLT) and checkpoint inhibitors (CPI). Ann Oncol 2020;31:S1156.
7. Loriot Y, Necchi A, Park SH, et al. BLC2001 Study Group. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med 2019;381:338-48.
8. Loehrer PJ Sr, Einhorn LH, Elson PJ, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 1992;10:1066-73.
9. Logothetis CJ, Dexeus FH, Finn L, et al. A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J Clin Oncol 1990;8:1050-5.
10. von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000;18:3068-77.
11. Galsky MD, Arija JÁA, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1547-57.
12. Powles T, Csőszi T, Özgüroğlu M, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:931-45.
13. Powles T, van der Heijden MS, Castellano D, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 2020;21:1574-88.
14. Rosenberg JE, Flaig TW, Friedlander TW, et al. Study EV-103: durability results of enfortumab vedotin plus pembrolizumab for locally advanced or metastatic urothelial carcinoma. J Clin Oncol 2020;38:5044.
15. Li X, Heyer WD. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res 2008;18:99-113.
16. Reardon JT, Vaisman A, Chaney SG, Sancar A. Efficient nucleotide excision repair of cisplatin, oxaliplatin, and bis-aceto-ammine-dichloro-cyclohexylamine-platinum(IV) (JM216) platinum intrastrand DNA diadducts. Cancer Res 1999;59:3968-71.
17. Lord RVN, Brabender J, Gandara D, et al. Low ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin Cancer Res 2002;8:2286-91.
18. Britten RA, Liu D, Tessier A, Hutchison MJ, Murray D. ERCC1 expression as a molecular marker of cisplatin resistance in human cervical tumor cells. Int J Cancer 2000;89:453-7.
19. Olaussen KA, Dunant A, Fouret P, et al. IALT Bio Investigators. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med 2006;355:983-91.
20. Dabholkar M, Vionnet J, Bostick-Bruton F, Yu JJ, Reed E. Messenger RNA levels of XPAC and ERCC1 in ovarian cancer tissue correlate with response to platinum-based chemotherapy. J Clin Invest 1994;94:703-8.
21. Metzger R, Leichman CG, Danenberg KD, et al. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol 1998;16:309-16.
22. Shirota Y, Stoehlmacher J, Brabender J, et al. ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J Clin Oncol 2001;19:4298-304.
23. Klatte T, Seitz C, Rink M, et al. ERCC1 as a prognostic and predictive biomarker for urothelial carcinoma of the bladder following radical cystectomy. J Urol 2015;194:1456-62.
24. Necchi A, Lo Vullo S, Raggi D, et al. Neoadjuvant sorafenib, gemcitabine, and cisplatin administration preceding cystectomy in patients with muscle-invasive urothelial bladder carcinoma: an open-label, single-arm, single-center, phase 2 study. Urol Oncol 2018;36:8.e1-8.
25. Hemdan T, Segersten U, Malmström P. 122 ERCC1-negative tumors benefit from neoadjuvant cisplatin-based chemotherapy whereas patients with ERCC1-positive tumors do not - results from a cystectomy trial database. Eur Urol 2014;13:e122.
26. Choueiri TK, Jacobus S, Bellmunt J, et al. Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol 2014;32:1889-94.
27. Sakano S, Ogawa S, Yamamoto Y, et al. ERCC1 and XRCC1 expression predicts survival in bladder cancer patients receiving combined trimodality therapy. Mol Clin Oncol 2013;1:403-10.
28. Sun JM, Sung JY, Park SH, et al. ERCC1 as a biomarker for bladder cancer patients likely to benefit from adjuvant chemotherapy. BMC Cancer 2012;12:187.
29. Kawashima A, Takayama H, Kawamura N, et al. Co-expression of ERCC1 and Snail is a prognostic but not predictive factor of cisplatin-based neoadjuvant chemotherapy for bladder cancer. Oncol Lett 2012;4:15-21.
30. Ozcan MF, Dizdar O, Dincer N, et al. Low ERCC1 expression is associated with prolonged survival in patients with bladder cancer receiving platinum-based neoadjuvant chemotherapy. Urol Oncol 2013;31:1709-15.
31. Nikitas N, Karadimou A, Tsitoura E, et al. Association of ERCC1 SNPs with outcome in platinum-treated patients with advanced urothelial cancer: a Hellenic Cooperative Oncology Group study. Pharmacogenomics 2012;13:1595-607.
32. Kim KH, Do IG, Kim HS, et al. Excision repair cross-complementation group 1 (ERCC1) expression in advanced urothelial carcinoma patients receiving cisplatin-based chemotherapy. APMIS 2010;118:941-8.
33. Hoffmann AC, Wild P, Leicht C, et al. MDR1 and ERCC1 expression predict outcome of patients with locally advanced bladder cancer receiving adjuvant chemotherapy. Neoplasia 2010;12:628-36.
34. Bellmunt J, Paz-Ares L, Cuello M, et al. Spanish Oncology Genitourinary Group. Gene expression of ERCC1 as a novel prognostic marker in advanced bladder cancer patients receiving cisplatin-based chemotherapy. Ann Oncol 2007;18:522-8.
35. Urun Y, Leow JJ, Fay AP, Albiges L, Choueiri TK, Bellmunt J. ERCC1 as a prognostic factor for survival in patients with advanced urothelial cancer treated with platinum based chemotherapy: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2017;120:120-6.
36. Robertson AG, Kim J, Al-Ahmadie H, et al. TCGA Research Network. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 2017;171:540-56.e25.
37. Li Q, Damish AW, Frazier Z, et al. ERCC2 helicase domain mutations confer nucleotide excision repair deficiency and drive cisplatin sensitivity in muscle-invasive bladder cancer. Clin Cancer Res 2019;25:977-88.
38. Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov 2014;4:1140-53.
39. Liu D, Plimack ER, Hoffman-Censits J, et al. Clinical validation of chemotherapy response biomarker ERCC2 in muscle-invasive urothelial bladder carcinoma. JAMA Oncol 2016;2:1094-6.
40. Christensen E, Birkenkamp-Demtröder K, Sethi H, et al. Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. J Clin Oncol 2019;37:1547-57.
41. Groenendijk FH, de Jong J, Fransen van de Putte EE, et al. ERBB2 mutations characterize a subgroup of muscle-invasive bladder cancers with excellent response to neoadjuvant chemotherapy. Eur Urol 2016;69:384-8.
42. Groenendijk FH, Fransen van de Putte EE, van Rhijn BW, Bernards R, van der Heijden MS. Reply to Eliezer M. Van Allen, Levi A. Garraway and Jonathan E. Rosenberg's Letter to the Editor re: Floris H. Groenendijk, Jeroen de Jong, Elisabeth E. Fransen van de Putte, et al. ERBB2 mutations characterize a subgroup of muscle-invasive bladder cancers with excellent response to neoadjuvant chemotherapy. Eur Urol. In press. http://dx.doi.org/10.1016/j.eururo.2015.01.014. Eur Urol 2015;68:e33-4.
43. Taber A, Christensen E, Lamy P, et al. Molecular correlates of cisplatin-based chemotherapy response in muscle invasive bladder cancer by integrated multi-omics analysis. Nat Commun 2020;11:4858.
44. Kim J, Mouw KW, Polak P, et al. Somatic ERCC2 mutations are associated with a distinct genomic signature in urothelial tumors. Nat Genet 2016;48:600-6.
45. Galsky MD, Daneshmand S, Chan KG, et al. Phase 2 trial of gemcitabine, cisplatin, plus nivolumab with selective bladder sparing in patients with muscle- invasive bladder cancer (MIBC): HCRN GU 16-257. J Clin Oncol 2021;39:4503.
46. Yang D, Khan S, Sun Y, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA 2011;306:1557-65.
47. Sakai W, Swisher EM, Karlan BY, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature 2008;451:1116-20.
48. Tutt A, Tovey H, Cheang MCU, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med 2018;24:628-37.
49. Carlo MI, Ravichandran V, Srinavasan P, et al. Cancer susceptibility mutations in patients with urothelial malignancies. J Clin Oncol 2020;38:406-14.
50. Nassar AH, Abou Alaiwi S, AlDubayan SH, et al. Prevalence of pathogenic germline cancer risk variants in high-risk urothelial carcinoma. Genet Med 2020;22:709-18.
51. Mullane SA, Werner L, Guancial EA, et al. Expression levels of DNA damage repair proteins are associated with overall survival in platinum-treated advanced urothelial carcinoma. Clin Genitourin Cancer 2016;14:352-9.
52. Lord CJ, Ashworth A. RAD51, BRCA2 and DNA repair: a partial resolution. Nat Struct Mol Biol 2007;14:461-2.
53. Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur Urol 2015;68:959-67.
54. Miron B, Ross EA, Anari F, et al. Defects in DNA repair genes and long-term survival in cisplatin-based neoadjuvant chemotherapy for muscle invasive bladder cancer (MIBC). J Clin Oncol 2019;37:4536-4536.
55. Teo MY, Bambury RM, Zabor EC, et al. DNA damage response and repair gene alterations are associated with improved survival in patients with platinum-treated advanced urothelial carcinoma. Clin Cancer Res 2017;23:3610-8.
56. Blaveri E, Simko JP, Korkola JE, et al. Bladder cancer outcome and subtype classification by gene expression. Clin Cancer Res 2005;11:4044-55.
57. Dyrskjøt L, Thykjaer T, Kruhøffer M, et al. Identifying distinct classes of bladder carcinoma using microarrays. Nat Genet 2003;33:90-6.
58. Lindgren D, Frigyesi A, Gudjonsson S, et al. Combined gene expression and genomic profiling define two intrinsic molecular subtypes of urothelial carcinoma and gene signatures for molecular grading and outcome. Cancer Res 2010;70:3463-72.
59. Sjödahl G, Lauss M, Lövgren K, et al. A molecular taxonomy for urothelial carcinoma. Clin Cancer Res 2012;18:3377-86.
60. Volkmer JP, Sahoo D, Chin RK, et al. Three differentiation states risk-stratify bladder cancer into distinct subtypes. Proc Natl Acad Sci U S A 2012;109:2078-83.
61. Tan TZ, Rouanne M, Tan KT, Huang RY, Thiery JP. Molecular subtypes of urothelial bladder cancer: results from a meta-cohort analysis of 2411 tumors. Eur Urol 2019;75:423-32.
62. Hurst CD, Alder O, Platt FM, et al. Genomic subtypes of non-invasive bladder cancer with distinct metabolic profile and female gender bias in KDM6A mutation frequency. Cancer Cell 2017;32:701-15.e7.
63. Hedegaard J, Lamy P, Nordentoft I, et al. Comprehensive transcriptional analysis of early-stage urothelial carcinoma. Cancer Cell 2016;30:27-42.
64. Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014;25:152-65.
65. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014;507:315-22.
66. Mo Q, Nikolos F, Chen F, et al. Prognostic power of a tumor differentiation gene signature for bladder urothelial carcinomas. J Natl Cancer Inst 2018;110:448-59.
67. Sjödahl G, Eriksson P, Liedberg F, Höglund M. Molecular classification of urothelial carcinoma: global mRNA classification versus tumour-cell phenotype classification. J Pathol 2017;242:113-25.
68. Marzouka NA, Eriksson P, Rovira C, Liedberg F, Sjödahl G, Höglund M. A validation and extended description of the Lund taxonomy for urothelial carcinoma using the TCGA cohort. Sci Rep 2018;8:3737.
69. Rebouissou S, Bernard-Pierrot I, de Reyniès A, et al. EGFR as a potential therapeutic target for a subset of muscle-invasive bladder cancers presenting a basal-like phenotype. Sci Transl Med 2014;6:244ra91.
70. Damrauer JS, Hoadley KA, Chism DD, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A 2014;111:3110-5.
71. Kamoun A, de Reyniès A, Allory Y, et al. Bladder Cancer Molecular Taxonomy Group. A consensus molecular classification of muscle-invasive bladder cancer. Eur Urol 2020;77:420-33.
72. McConkey DJ, Choi W, Shen Y, et al. A prognostic gene expression signature in the molecular classification of chemotherapy-naïve urothelial cancer is predictive of clinical outcomes from neoadjuvant chemotherapy: a phase 2 trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with bevacizumab in urothelial cancer. Eur Urol 2016;69:855-62.
73. Seiler R, Ashab HAD, Erho N, et al. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur Urol 2017;72:544-54.
74. Smith SC, Baras AS, Lee JK, Theodorescu D. The COXEN principle: translating signatures of in vitro chemosensitivity into tools for clinical outcome prediction and drug discovery in cancer. Cancer Res 2010;70:1753-8.
75. Flaig TW, Tangen CM, Daneshmand S, et al. A randomized phase II study of coexpression extrapolation (COXEN) with neoadjuvant chemotherapy for bladder cancer (SWOG S1314; NCT02177695). Clin Cancer Res 2021;27:2435-41.
76. Rosenberg JE, Ballman KV, Halabi S, et al. CALGB 90601 (Alliance): randomized, double-blind, placebo-controlled phase III trial comparing gemcitabine and cisplatin with bevacizumab or placebo in patients with metastatic urothelial carcinoma. J Clin Oncol 2019;37:4503.
77. National Cancer Institute (NCI). A randomized double-blinded phase III study comparing gemcitabine, cisplatin, and bevacizumab to gemcitabine, cisplatin, and placebo in patients with advanced transitional cell carcinoma. Available from: https://clinicaltrials.gov/ct2/show/NCT00942331 [Last accessed on 11 Jan 2022].
78. Geynisman DM, Abbosh P, Ross EA, et al. A phase II trial of risk enabled therapy after initiating neoadjuvant chemotherapy for bladder cancer (RETAIN BLADDER): interim analysis. J Clin Oncol 2021;39:397.
79. Rosenberg JE, Hoffman-censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20.
80. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67-76.
81. Sharma P, Retz M, Siefker-radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312-22.
82. Balar AV, Castellano D, O'donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18:1483-92.
83. Powles T, O'Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 Open-label study. JAMA Oncol 2017;3:e172411.
84. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2018;19:51-64.
85. Grivas P, Park SH, Voog E, et al. Avelumab first-line (1L) maintenance plus best supportive care (BSC) versus BSC alone for advanced urothelial carcinoma (UC): analysis of time to end of next-line therapy in JAVELIN Bladder 100. J Clin Oncol 2021;39:4525.
86. Powles T, Loriot Y, Bellmunt J, et al. 699O avelumab first-line (1L) maintenance + best supportive care (BSC) vs BSC alone for advanced urothelial carcinoma (UC): association between clinical outcomes and exploratory biomarkers. Ann Oncol 2020;31:S552-3.
87. Powles T, Assaf ZJ, Davarpanah N, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature 2021;595:432-7.
88. Bellmunt J, Hussain M, Gschwend JE, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2021;22:525-37.
89. Wang L, Gong Y, Saci A, et al. Fibroblast growth factor receptor 3 alterations and response to PD-1/PD-L1 blockade in patients with metastatic urothelial cancer. Eur Urol 2019;76:599-603.
90. Rose TL, Weir WH, Mayhew GM, et al. Fibroblast growth factor receptor 3 alterations and response to immune checkpoint inhibition in metastatic urothelial cancer: a real world experience. Br J Cancer 2021;125:1251-60.
91. Powles T, Carroll D, Chowdhury S, et al. An adaptive, biomarker-directed platform study of durvalumab in combination with targeted therapies in advanced urothelial cancer. Nat Med 2021;27:793-801.
92. FDA alerts health care professionals and oncology clinical investigators about an efficacy issue identified in clinical trials for some patients taking keytruda (pembrolizumab) or tecentriq (atezolizumab) as monotherapy to treat urothelial cancer with low expression of PD-L1. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-alerts-health-care-professionals-and-oncology-clinical-investigators-about-efficacy-issue [Last accessed on 11 Jan 2022].
93. Galsky MD, Necchi A, Sridhar SS, et al. A phase III, randomized, open-label, multicenter, global study of first-line durvalumab plus standard of care (SoC) chemotherapy and durvalumab plus tremelimumab, and SoC chemotherapy versus SoC chemotherapy alone in unresectable locally advanced or metastatic urothelial cancer (NILE). J Clin Oncol 2021;39:TPS504.
94. Rui X, Gu TT, Pan HF, Zhang HZ. Evaluation of PD-L1 biomarker for immune checkpoint inhibitor (PD-1/PD-L1 inhibitors) treatments for urothelial carcinoma patients: a meta-analysis. Int Immunopharmacol 2019;67:378-85.
95. Litchfield K, Reading JL, Puttick C, et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell 2021;184:596-614.e14.
96. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018;391:748-57.
97. Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol 2017;12:208-22.
98. Ratcliffe MJ, Sharpe A, Midha A, et al. Agreement between programmed cell death ligand-1 diagnostic assays across multiple protein expression cutoffs in non-small cell lung cancer. Clin Cancer Res 2017;23:3585-91.
99. Tsao MS, Kerr KM, Kockx M, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol 2018;13:1302-11.
100. Decazes P, Bohn P. Immunotherapy by immune checkpoint inhibitors and nuclear medicine imaging: current and future applications. Cancers (Basel) 2020;12:371.
101. Bensch F, van der Veen EL, Lub-de Hooge MN, et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med 2018;24:1852-8.
102. Niemeijer AN, Leung D, Huisman MC, et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun 2018;9:4664.
103. Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Australian Pancreatic Cancer Genome Initiative, ICGC Breast Cancer Consortium, ICGC MMML-Seq Consortium, ICGC PedBrain. Signatures of mutational processes in human cancer. Nature 2013;500:415-21.
104. Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019;51:202-6.
105. Mariathasan S, Turley SJ, Nickles D, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554:544-8.
106. Bellmunt J, de Wit R, Fradet Y, et al. 747P association of TMB with efficacy of pembrolizumab (pembro) in patients (pts) with advanced urothelial cancer (UC): results from KEYNOTE-045 and KEYNOTE-052. Ann Oncol 2020;31:S580-1.
107. FDA approves pembrolizumab for adults and children with TMB-H solid tumors. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors [Last accessed on 11 Jan 2022].
108. Yarchoan M, Albacker LA, Hopkins AC, et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight 2019;4:126908.
109. Galsky MD, Saci A, Szabo PM, et al. Nivolumab in patients with advanced platinum-resistant urothelial carcinoma: efficacy, safety, and biomarker analyses with extended follow-up from checkmate 275. Clin Cancer Res 2020;26:5120-8.
110. Galsky MD, Banchereau R, Hamidi HR, et al. Tumor, immune, and stromal characteristics associated with clinical outcomes with atezolizumab (atezo) + platinum-based chemotherapy (PBC) or atezo monotherapy (mono) versus PBC in metastatic urothelial cancer (mUC) from the phase III IMvigor130 study. J Clin Oncol 2020;38:5011.
111. Valero C, Lee M, Hoen D, et al. Response rates to anti-PD-1 Immunotherapy in microsatellite-stable solid tumors with 10 or more mutations per megabase. JAMA Oncol 2021;7:739-43.
112. Vokes NI, Liu D, Ricciuti B, et al. Harmonization of tumor mutational burden quantification and association with response to immune checkpoint blockade in non-small-cell lung cancer. JCO Precis Oncol 2019;3:1-12.
113. Mandal R, Samstein RM, Lee KW, et al. Genetic diversity of tumors with mismatch repair deficiency influences anti-PD-1 immunotherapy response. Science 2019;364:485-91.
114. McGranahan N, Rosenthal R, Hiley CT, et al. TRACERx Consortium. Allele-specific HLA loss and immune escape in lung cancer evolution. Cell 2017;171:1259-71.e11.
115. Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS Jr. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 1998;281:1680-3.
116. Vredevoogd DW, Kuilman T, Ligtenberg MA, et al. Augmenting immunotherapy impact by lowering tumor TNF cytotoxicity threshold. Cell 2019;178:585-99.e15.
117. Zhang J, Bu X, Wang H, et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature 2018;553:91-5.
118. O'donnell PH, Grivas P, Balar AV, et al. Biomarker findings and mature clinical results from KEYNOTE-052: first-line pembrolizumab (pembro) in cisplatin-ineligible advanced urothelial cancer (UC). J Clin Oncol 2017;35:4502.
119. Wang L, Saci A, Szabo PM, et al. EMT- and stroma-related gene expression and resistance to PD-1 blockade in urothelial cancer. Nat Commun 2018;9:3503.
120. Calon A, Lonardo E, Berenguer-Llergo A, et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet 2015;47:320-9.
122. Lin RL, Zhao LJ. Mechanistic basis and clinical relevance of the role of transforming growth factor-β in cancer. Cancer Biol Med 2015;12:385-93.
123. Powles T, Petrylak DP, Park SH, et al. Avelumab first-line (1L) maintenance for advanced urothelial carcinoma (UC): analysis of clinical and genomic subgroups from the JAVELIN Bladder 100 trial. J Clin Oncol 2021;39:4520.
124. Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 2018;24:541-50.
125. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1-10.
126. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7.
127. Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA. Profiling Tumor Infiltrating Immune Cells with CIBERSORT. Methods Mol Biol 2018;1711:243-59.
128. Aran D, Hu Z, Butte AJ. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol 2017;18:220.
129. Cao J, Yang X, Li J, et al. Screening and identifying immune-related cells and genes in the tumor microenvironment of bladder urothelial carcinoma: based on TCGA database and bioinformatics. Front Oncol 2019;9:1533.
130. Gohil SH, Iorgulescu JB, Braun DA, Keskin DB, Livak KJ. Applying high-dimensional single-cell technologies to the analysis of cancer immunotherapy. Nat Rev Clin Oncol 2021;18:244-56.
131. Guruprasad P, Lee YG, Kim KH, Ruella M. The current landscape of single-cell transcriptomics for cancer immunotherapy. J Exp Med 2021;218:e20201574.
132. Oh DY, Kwek SS, Raju SS, et al. Intratumoral CD4+ T cells mediate anti-tumor cytotoxicity in human bladder cancer. Cell 2020;181:1612-25.e13.
133. Chen Z, Zhou L, Liu L, et al. Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat Commun 2020;11:5077.
134. Sfakianos JP, Daza J, Hu Y, et al. Epithelial plasticity can generate multi-lineage phenotypes in human and murine bladder cancers. Nat Commun 2020;11:2540.
135. Mota JM, Leite CA, Souza LE, et al. Post-sepsis state induces tumor-associated macrophage accumulation through CXCR4/CXCL12 and favors tumor progression in mice. Cancer Immunol Res 2016;4:312-22.
136. Wang L, Sfakianos JP, Beaumont KG, et al. Myeloid cell-associated resistance to PD-1/PD-L1 blockade in urothelial cancer revealed through bulk and single-cell RNA sequencing. Clin Cancer Res 2021;27:4287-300.
137. Siefker-radtke AO, Necchi A, Park SH, et al. BLC2001 Study Group. ERDAFITINIB in locally advanced or metastatic urothelial carcinoma (mUC): long-term outcomes in BLC2001. J Clin Oncol 2020;38:5015.
138. Challita-Eid PM, Satpayev D, Yang P, et al. Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res 2016;76:3003-13.
139. Doronina SO, Toki BE, Torgov MY, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol 2003;21:778-84.
140. Mathijssen RH, van Alphen RJ, Verweij J, et al. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin Cancer Res 2001;7:2182-94.
141. Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem 2011;149:121-30.
142. Plotnikov AN, Schlessinger J, Hubbard SR, Mohammadi M. Structural basis for FGF receptor dimerization and activation. Cell 1999;98:641-50.
143. Dieci MV, Arnedos M, Andre F, Soria JC. Fibroblast growth factor receptor inhibitors as a cancer treatment: from a biologic rationale to medical perspectives. Cancer Discov 2013;3:264-79.
144. Tomlinson DC, Baldo O, Harnden P, Knowles MA. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J Pathol 2007;213:91-8.
145. di Martino E, Tomlinson DC, Knowles MA. A decade of FGF receptor research in bladder cancer: past, present, and future challenges. Adv Urol 2012;2012:429213.
146. Helsten T, Elkin S, Arthur E, Tomson BN, Carter J, Kurzrock R. The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin Cancer Res 2016;22:259-67.
147. Costa R, Carneiro BA, Taxter T, et al. FGFR3-TACC3 fusion in solid tumors: mini review. Oncotarget 2016;7:55924-38.
148. Siefker-radtke A, Loriot Y, Siena S, et al. 752P Updated data from the NORSE trial of erdafitinib (ERDA) plus cetrelimab (CET) in patients (pts) with metastatic or locally advanced urothelial carcinoma (mUC) and specific fibroblast growth factor receptor (FGFR) alterations. Ann Oncol 2020;31:S584-5.
149. Pal SK, Rosenberg JE, Hoffman-Censits JH, et al. Efficacy of BGJ398, a fibroblast growth factor receptor 1-3 inhibitor, in patients with previously treated advanced urothelial carcinoma with FGFR3 alterations. Cancer Discov 2018;8:812-21.
150. Bellmunt J, Picus J, Kohli M, et al. FIERCE-21: Phase 1b/2 study of docetaxel + b-701, a selective inhibitor of FGFR3, in relapsed or refractory (R/R) metastatic urothelial carcinoma (mUCC). J Clin Oncol 2018;36:4534.
151. Necchi A, Castellano DE, Mellado B, et al. Fierce-21: Phase II study of vofatmab (B-701), a selective inhibitor of FGFR3, as salvage therapy in metastatic urothelial carcinoma (mUC). J Clin Oncol 2019;37:409.
152. Siefker-radtke AO, Currie G, Abella E, et al. FIERCE-22: clinical activity of vofatamab (V) a FGFR3 selective inhibitor in combination with pembrolizumab (P) in WT metastatic urothelial carcinoma, preliminary analysis. J Clin Oncol 2019;37:4511.
153. Abdul-karim RM, Chaudhry A, Patrikidou A, et al. Derazantinib (DZB) in combination with atezolizumab (AZB) in patients with solid tumors: results from the dose-finding phase Ib substudy of FIDES-02. J Clin Oncol 2021;39:437.
154. Chaudhry A, Sternberg CN, De Santis M, et al. FIDES-02, a phase Ib/II study of derazantinib (DZB) as monotherapy and combination therapy with atezolizumab (A) in patients with surgically unresectable or metastaticurothelial cancer (UC) and FGFR genetic aberrations. J Clin Oncol 2020;38:TPS590-TPS590.
155. Hyman DM, Goyal L, Grivas P, et al. FUZE clinical trial: a phase 2 study of Debio 1347 in FGFR fusion-positive advanced solid tumors irrespectively of tumor histology. J Clin Oncol 2019;37:TPS3157.
156. Meric-Bernstam F, Goyal L, Tran B, et al. Abstract CT238: TAS-120 in patients with advanced solid tumors bearing FGF/FGFR aberrations: a phase I study. Cancer Res 2019;79:CT238.
157. Milowsky MI, Dittrich C, Durán I, et al. Phase 2 trial of dovitinib in patients with progressive FGFR3-mutated or FGFR3 wild-type advanced urothelial carcinoma. Eur J Cancer 2014;50:3145-52.
158. Quinn DI, Petrylak DP, Bellmunt J, et al. FORT-1: phase II/III study of rogaratinib versus chemotherapy (CT) in patients (pts) with locally advanced or metastatic urothelial carcinoma (UC) selected based on FGFR1/3 mRNA expression. J Clin Oncol 2020;38:489.
159. Rosenberg JE, Gajate P, Morales-barrera R, et al. Safety and preliminary efficacy of rogaratinib in combination with atezolizumab in a phase Ib/II study (FORT-2) of first-line treatment in cisplatin-ineligible patients (pts) with locally advanced or metastatic urothelial cancer (UC) and FGFR mRNA overexpression. J Clin Oncol 2020;38:5014.
160. Yue S, Li Y, Chen X, et al. FGFR-TKI resistance in cancer: current status and perspectives. J Hematol Oncol 2021;14:23.
161. Guagnano V, Furet P, Spanka C, et al. Discovery of 3-(2,6-dichloro-3,5-dimethoxy-phenyl)-1-{6-[4-(4-ethyl-piperazin-1-yl)-phenylamino]-pyrimidin-4-yl}-1-methyl-urea (NVP-BGJ398), a potent and selective inhibitor of the fibroblast growth factor receptor family of receptor tyrosine kinase. J Med Chem 2011;54:7066-83.
162. Goyal L, Saha SK, Liu LY, et al. Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion-positive cholangiocarcinoma. Cancer Discov 2017;7:252-63.
163. Datta J, Damodaran S, Parks H, et al. Akt activation mediates acquired resistance to fibroblast growth factor receptor inhibitor BGJ398. Mol Cancer Ther 2017;16:614-24.
164. Wang L, Šuštić T, Leite de Oliveira R, et al. A functional genetic screen identifies the phosphoinositide 3-kinase pathway as a determinant of resistance to fibroblast growth factor receptor inhibitors in FGFR mutant urothelial cell carcinoma. Eur Urol 2017;71:858-62.
165. Ryan MR, Sohl CD, Luo B, Anderson KS. The FGFR1 V561M gatekeeper mutation drives AZD4547 resistance through STAT3 activation and EMT. Mol Cancer Res 2019;17:532-43.
166. Mandai K, Rikitake Y, Mori M, Takai Y. Nectins and nectin-like molecules in development and disease. Curr Top Dev Biol 2015;112:197-231,.
167. Rosenberg J, Sridhar SS, Zhang J, et al. EV-101: a phase i study of single-agent enfortumab vedotin in patients with nectin-4-positive solid tumors, including metastatic urothelial carcinoma. J Clin Oncol 2020;38:1041-9.
168. Chu CE, Sjöström M, Egusa EA, et al. Heterogeneity in NECTIN4 expression across molecular subtypes of urothelial cancer mediates sensitivity to enfortumab vedotin. Clin Cancer Res 2021;27:5123-30.
169. Hoffman-Censits JH, Lombardo KA, Parimi V, et al. Expression of nectin-4 in bladder urothelial carcinoma, in morphologic variants, and nonurothelial histotypes. Appl Immunohistochem Mol Morphol 2021;29:619-25.
170. Hoimes CJ, Rosenberg JE, Petrylak DP, et al. Study EV-103: new cohorts testing enfortumab vedotin alone or in combination with pembrolizumab in muscle invasive urothelial cancer. J Clin Oncol 2020;38:TPS595.
171. Rosenberg JE, Flaig TW, Friedlander TW, et al. Study EV-103: preliminary durability results of enfortumab vedotin plus pembrolizumab for locally advanced or metastatic urothelial carcinoma. J Clin Oncol 2020;38:441.
172. Rapani E, Sacchetti A, Corda D, Alberti S. Human TROP-2 is a tumor-associated calcium signal transducer. Int J Cancer 1998;76:671-6.
173. Faltas B, Goldenberg DM, Ocean AJ, et al. Sacituzumab govitecan, a novel antibody--drug conjugate, in patients with metastatic platinum-resistant urothelial carcinoma. Clin Genitourin Cancer 2016;14:e75-9.
174. Zhang L, Yang G, Jiang H, et al. TROP2 is associated with the recurrence of patients with non-muscle invasive bladder cancer. Int J Clin Exp Med 2017;10:1643-50.
175. Wang X, Long M, Dong K, et al. Chemotherapy agents-induced immunoresistance in lung cancer cells could be reversed by trop-2 inhibition in vitro and in vivo by interaction with MAPK signaling pathway. Cancer Biol Ther 2013;14:1123-32.
176. Guerra E, Trerotola M, Tripaldi R, et al. Trop-2 induces tumor growth through AKT and determines sensitivity to AKT inhibitors. Clin Cancer Res 2016;22:4197-205.
177. Vidula N, Yau C, Rugo HS. Trop2 gene expression (Trop2e) in primary breast cancer (BC): correlations with clinical and tumor characteristics. J Clin Oncol 2017;35:1075.
178. Hurvitz SA, Tolaney SM, Punie K, et al. Abstract GS3-06: biomarker evaluation in the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Cancer Res 2021;81:GS3-6.
179. Grivas P, Tagawa ST, Bellmunt J, et al. TROPiCS-04: study of sacituzumab govitecan in metastatic or locally advanced unresectable urothelial cancer that has progressed after platinum and checkpoint inhibitor therapy. J Clin Oncol 2021;39:TPS498.
180. Drakaki A, Rezazadeh Kalebasty A, Lee J, et al. Phase Ib/II umbrella trial to evaluate the safety and efficacy of multiple 2L cancer immunotherapy (CIT) combinations in advanced/metastatic urothelial carcinoma (mUC): MORPHEUS-mUC. J Clin Oncol 2020;38:TPS591.
181. Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int 2014;2014:852748.
182. Coogan CL, Estrada CR, Kapur S, Bloom KJ. HER-2/neu protein overexpression and gene amplification in human transitional cell carcinoma of the bladder. Urology 2004;63:786-90.
183. Latif Z, Watters AD, Dunn I, Grigor KM, Underwood MA, Bartlett JM. HER2/neu overexpression in the development of muscle-invasive transitional cell carcinoma of the bladder. Br J Cancer 2003;89:1305-9.
184. Hussain MH, MacVicar GR, Petrylak DP, et al. National Cancer Institute. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter phase II National Cancer Institute trial. J Clin Oncol 2007;25:2218-24.
185. Oudard S, Culine S, Vano Y, et al. Multicentre randomised phase II trial of gemcitabine+platinum, with or without trastuzumab, in advanced or metastatic urothelial carcinoma overexpressing Her2. Eur J Cancer 2015;51:45-54.
186. Powles T, Huddart RA, Elliott T, et al. Phase III, double-blind, randomized trial that compared maintenance lapatinib versus placebo after first-line chemotherapy in patients with human epidermal growth factor receptor 1/2-positive metastatic bladder cancer. J Clin Oncol 2017;35:48-55.
187. Choudhury NJ, Campanile A, Antic T, et al. Afatinib activity in platinum-refractory metastatic urothelial carcinoma in patients with ERBB alterations. J Clin Oncol 2016;34:2165-71.
188. Font Pous A, Puente J, Castellano DE, et al. Phase II trial of afatinib in patients with advanced/metastatic urothelial carcinoma (UC) with genetic alterations in ERBB receptors 1-3 who failed on platinum-based chemotherapy (CT). J Clin Oncol 2018;36:TPS540-TPS540.
189. Hainsworth JD, Meric-Bernstam F, Swanton C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol 2018;36:536-42.
190. Sheng X, Zhou A, Yao X, et al. A phase II study of RC48-ADC in HER2-positive patients with locally advanced or metastatic urothelial carcinoma. J Clin Oncol 2019;37:4509.
191. Li BT, Makker V, Buonocore DJ, et al. A multi-histology basket trial of ado-trastuzumab emtansine in patients with HER2 amplified cancers. J Clin Oncol 2018;36:2502.
192. Duan Y, Haybaeck J, Yang Z. Therapeutic potential of PI3K/AKT/mTOR pathway in gastrointestinal stromal tumors: rationale and progress. Cancers (Basel) 2020;12:2972.
193. Sathe A, Nawroth R. Targeting the PI3K/AKT/mTOR pathway in bladder cancer. Methods Mol Biol 2018;1655:335-50,.
194. Iyer G, Al-Ahmadie H, Schultz N, et al. Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. J Clin Oncol 2013;31:3133-40.
195. Platt FM, Hurst CD, Taylor CF, Gregory WM, Harnden P, Knowles MA. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clin Cancer Res 2009;15:6008-17.
196. Calderaro J, Rebouissou S, de Koning L, et al. PI3K/AKT pathway activation in bladder carcinogenesis. Int J Cancer 2014;134:1776-84.
197. Cappellen D, Gil Diez de Medina S, Chopin D, Thiery JP, Radvanyi F. Frequent loss of heterozygosity on chromosome 10q in muscle-invasive transitional cell carcinomas of the bladder. Oncogene 1997;14:3059-66.
198. Aveyard JS, Skilleter A, Habuchi T, Knowles MA. Somatic mutation of PTEN in bladder carcinoma. Br J Cancer 1999;80:904-8.
199. Tsuruta H, Kishimoto H, Sasaki T, et al. Hyperplasia and carcinomas in Pten-deficient mice and reduced PTEN protein in human bladder cancer patients. Cancer Res 2006;66:8389-96.
200. Knowles MA, Habuchi T, Kennedy W, Cuthbert-Heavens D. Mutation spectrum of the 9q34 tuberous sclerosis gene TSC1 in transitional cell carcinoma of the bladder. Cancer Res 2003;63:7652-6.
201. Milowsky MI, Iyer G, Regazzi AM, et al. Phase II study of everolimus in metastatic urothelial cancer. BJU Int 2013;112:462-70.
202. Bellmunt J, Lalani AA, Jacobus S, et al. Everolimus and pazopanib (E/P) benefit genomically selected patients with metastatic urothelial carcinoma. Br J Cancer 2018;119:707-12.
203. Wagle N, Grabiner BC, Van Allen EM, et al. Activating mTOR mutations in a patient with an extraordinary response on a phase I trial of everolimus and pazopanib. Cancer Discov 2014;4:546-53.
204. Kim JW, Milowsky MI, Hahn NM, et al. Sapanisertib, a dual mTORC1/2 inhibitor, for TSC1- or TSC2- mutated metastatic urothelial carcinoma (mUC). J Clin Oncol 2021;39:431.
205. McPherson V, Reardon B, Bhayankara A, et al. A phase 2 trial of buparlisib in patients with platinum-resistant metastatic urothelial carcinoma. Cancer 2020;126:4532-44.
206. Flaherty KT, Gray RJ, Chen AP, et al. NCI-MATCH team. Molecular landscape and actionable alterations in a genomically guided cancer clinical trial: national cancer institute molecular analysis for therapy choice (NCI-MATCH). J Clin Oncol 2020;38:3883-94.
207. Sathe A, Guerth F, Cronauer MV, et al. Mutant PIK3CA controls DUSP1-dependent ERK 1/2 activity to confer response to AKT target therapy. Br J Cancer 2014;111:2103-13.
208. Dickstein RJ, Nitti G, Dinney CP, Davies BR, Kamat AM, McConkey DJ. Autophagy limits the cytotoxic effects of the AKT inhibitor AZ7328 in human bladder cancer cells. Cancer Biol Ther 2012;13:1325-38.
209. Seront E, Rottey S, Filleul B, et al. Phase II study of dual phosphoinositol-3-kinase (PI3K) and mammalian target of rapamycin (mTOR) inhibitor BEZ235 in patients with locally advanced or metastatic transitional cell carcinoma. BJU Int 2016;118:408-15.
210. Munster P, Aggarwal R, Hong D, et al. First-in-human phase I study of GSK2126458, an oral pan-class I phosphatidylinositol-3-kinase inhibitor, in patients with advanced solid tumor malignancies. Clin Cancer Res 2016;22:1932-9.
211. Sachdev E, Tabatabai R, Roy V, Rimel BJ, Mita MM. PARP inhibition in cancer: an update on clinical development. Target Oncol 2019;14:657-79.
212. Grivas P, Loriot Y, Feyerabend S, et al. Rucaparib for recurrent, locally advanced, or metastatic urothelial carcinoma (mUC): results from ATLAS, a phase II open-label trial. J Clin Oncol 2020;38:440.
213. Apolo AB, Nadal R, Tomita Y, et al. Cabozantinib in patients with platinum-refractory metastatic urothelial carcinoma: an open-label, single-centre, phase 2 trial. Lancet Oncol 2020;21:1099-109.
214. Apolo AB, Nadal R, Girardi DM, et al. Phase I study of cabozantinib and nivolumab alone or with ipilimumab for advanced or metastatic urothelial carcinoma and other genitourinary tumors. J Clin Oncol 2020;38:3672-84.
215. Necchi A, Giannatempo P, Raggi D, et al. Cabozantinib (CABO) plus durvalumab (DURVA) in patients with advanced and chemotherapy-treated bladder carcinoma of urothelial and non-urothelial histology: an open-label, single-arm, phase 2 trial. J Clin Oncol 2018;36:TPS536.
216. Pal SK, Vaishampayan UN, Castellano DE, et al. Phase Ib (COSMIC-021) trial of cabozantinib (C) in urothelial carcinoma (UC) or C in combination with atezolizumab (A) in patients (pts) with UC, castrate resistant prostate cancer (CRPC) or renal cell carcinoma (RCC). J Clin Oncol 2019;37:TPS683.
217. Petrylak DP, de Wit R, Chi KN, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): overall survival and updated results of a randomised, double-blind, phase 3 trial. Lancet Oncol 2020;21:105-20.
218. Msaouel P, Siefker-radtke A, Sweis R, et al. 705MO Sitravatinib (sitra) in combination with nivolumab (nivo) demonstrates clinical activity in checkpoint inhibitor (CPI) naïve, platinum-experienced patients (pts) with advanced or metastatic urothelial carcinoma (UC). Ann Oncol 2020;31:S556.
219. Cobain EF, Wu YM, Vats P, et al. Assessment of clinical benefit of integrative genomic profiling in advanced solid tumors. JAMA Oncol 2021;7:525-33.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Jun T, Anker J, Galsky MD. Biomarkers for therapy selection in metastatic urothelial cancer. J Cancer Metastasis Treat 2022;8:1. http://dx.doi.org/10.20517/2394-4722.2021.171
AMA Style
Jun T, Anker J, Galsky MD. Biomarkers for therapy selection in metastatic urothelial cancer. Journal of Cancer Metastasis and Treatment. 2022; 8: 1. http://dx.doi.org/10.20517/2394-4722.2021.171
Chicago/Turabian Style
Jun, Tomi, Jonathan Anker, Matthew D. Galsky. 2022. "Biomarkers for therapy selection in metastatic urothelial cancer" Journal of Cancer Metastasis and Treatment. 8: 1. http://dx.doi.org/10.20517/2394-4722.2021.171
ACS Style
Jun, T.; Anker J.; Galsky MD. Biomarkers for therapy selection in metastatic urothelial cancer. J. Cancer. Metastasis. Treat. 2022, 8, 1. http://dx.doi.org/10.20517/2394-4722.2021.171
About This Article
Copyright
Data & Comments
Data
 Cite This Article 65 clicks
Cite This Article 65 clicks


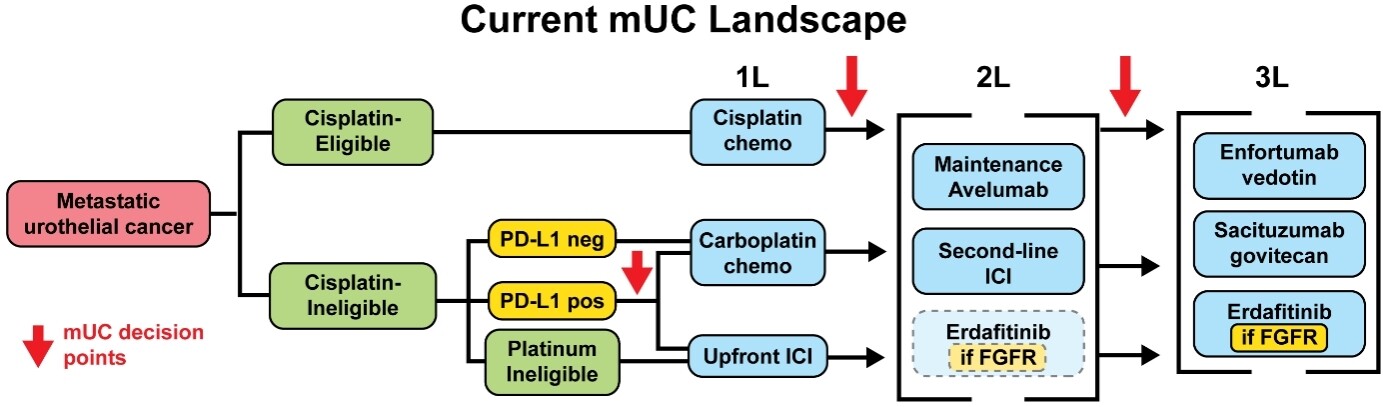
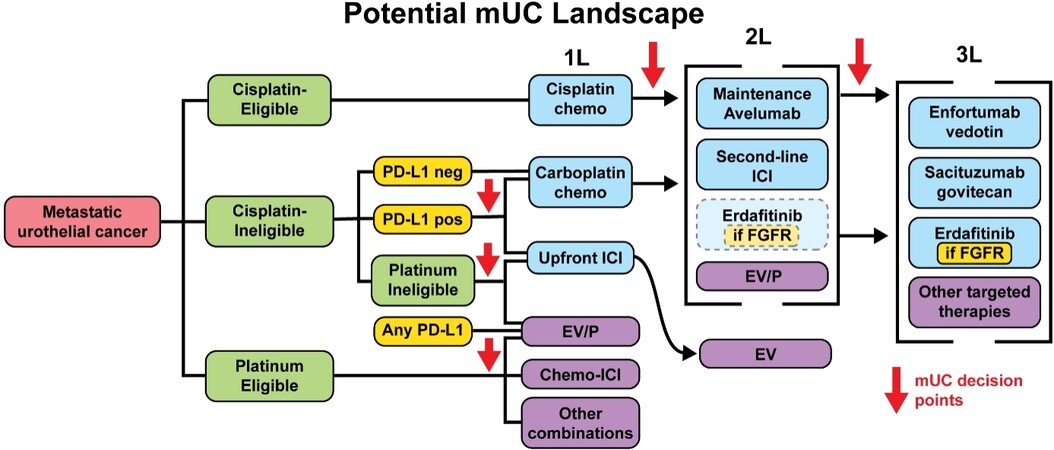
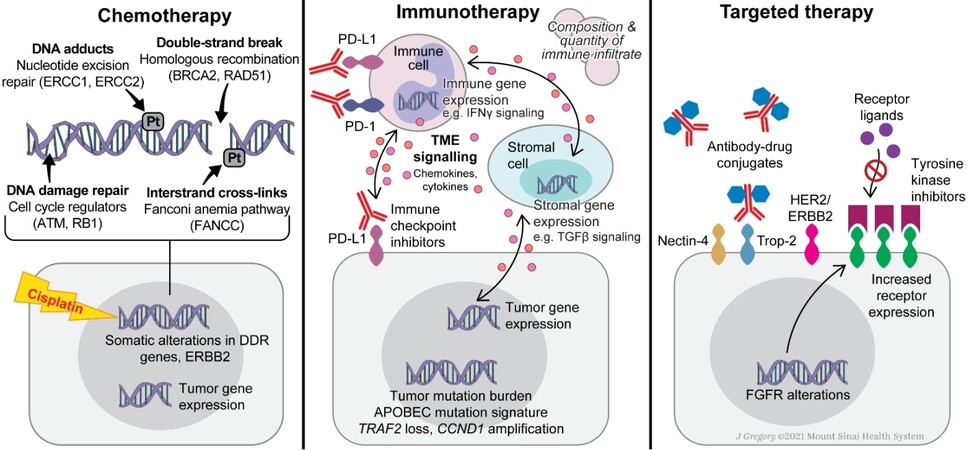









Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.