Thymic carcinoma: review and update
Abstract
Thymic carcinoma (TC) is a rare thymic epithelial neoplasm with an aggressive clinical course. There are many recognized histologic subtypes as described by the fifth edition of the World Health Organization (WHO) Classification of Thoracic Tumors; however, given the rarity of this tumor group, diagnosis remains a challenge, especially on limited tissue samples. Additionally, rare variants of TC are continuing to be established, particularly in the era of molecular diagnostics. Herein, histologic subtypes are described as the rare subtypes of TC in the context of their immunoprofile, cytogenetic or molecular features, clinical presentation, and ensuing challenges.
Keywords
INTRODUCTION
Thymic carcinoma (TC) is a rare thymic epithelial neoplasm (TEN) with heterogeneous features. Compared to thymomas, TC displays a more aggressive clinical course with high rates of metastases and recurrences. The estimated median overall survival rate between all stages is 6.6 years[1]. From a pathology perspective, TC does not have a specific unifying morphologic picture and can present with different histologic features. In general, TC shows greater architectural distortion and cytologic atypia when compared to thymomas. Immunohistochemistry can be beneficial in differentiating primary TC and metastatic disease; however, the final diagnosis is based on a careful pathologic, clinical, and radiographic correlation.
TC can present with locally invasive symptoms such as cough, chest pain, or superior vena cava syndrome. Red cell aplasia or dysglobulinemia occurs more commonly in thymoma, and is infrequent in TC[2]. Unlike thymomas, the presentation of paraneoplastic syndrome and myasthenia gravis has rarely been reported and if present, the diagnosis of TC is challenging as this is an active area of controversy[3,4].
The majority of the TC cases present with local invasion into adjacent mediastinal structures. In retrospective studies, metastases were described in 50%-65% of TC cases[5,6]. Even after surgical resection, TC behaves aggressively and shows frequent recurrences.
A unifying etiology of TC is currently unknown with the oncogenesis differing from subtype to subtype. As an example, NUT carcinoma, a rare thoracic cancer that is clinically aggressive and contains undifferentiated tumor cells, is characterized by a nuclear protein in testis (NUTM1) gene fusion and thymic mucoepidermoid carcinoma by the classic mastermind-like transcriptional coactivator 2 (MAML2) fusion[7]. Additionally, the loss of chromosome 16q in thymic squamous cell carcinoma has been described[8,9]. In rare scenarios, TC occurs with a coinciding thymoma, raising the possibility that the carcinoma may harbor an existing clonal relationship with the thymoma.
Multiple staging systems have been developed for thymomas and TC with much controversy over utility. The emphasis on the capsule of the Masaoka-Koga system does not translate to TC as they often do not present with a capsule. Previously, thymomas have been classified as malignant if there was an invasion into the capsule or demonstrated metastatic disease. In general, it is preferred to use the terms “thymoma” or “thymic carcinoma”, as “malignant thymoma type II” and “WHO type C thymoma” are no longer accepted terminology.
Clinical features
TC is an aggressive neoplasm that accounts for 14%-22% of all TEN[10]. It can occur in all age groups but is most frequent in adults with a mean age of 46 and a slight male predominance (1.5:1 male-to-female)[11]. Most patients present with symptoms related to the anterior mediastinal location of the mass, such as chest pain, shortness of breath, and superior vena cava syndrome. The tumors often present at advanced stages with an invasion of local anatomic structures and a significant proportion developing metastasis to lymph nodes and distant sites.
Diagnostic challenges
Differentiating between a metastasis to the anterior mediastinum and TC can be a diagnostic challenge[12,13]. The diagnosis is especially challenging when pathologists are confronted with limited tissue, such as a core biopsy. This can be the case when complete resection is not feasible due to age or underlying comorbidities of the patient.
As the differential diagnosis of anterior mediastinal masses is broad, cytokeratin staining is useful in differentiating between lymphoid neoplasms and carcinomas. Expression of c-KIT (CD117) can be a helpful marker in diagnosing TC, as positive immunoreactivity for c-KIT is more commonly seen with TC[14,15]. Similarly, CD5 expression can be seen in TC and only positive in a small subset of thymomas[16]. Table 1 provides a list of morphologic, immunohistochemical, and radiographic features that may assist in the differentiation between thymoma and TC.
Comparison between thymoma and thymic carcinoma
| Thymoma | Thymic carcinoma | |
| Cytologic features | Mild to moderate atypia | Marked atypia |
| Mitotic figures | Lower | More frequent |
| Lymphocytes | Perivascular spaces, immature T-cell phenotype | Mature T- and B-cells |
| Growth and necrosis | Lobular, usually lacks vascular invasion and necrosis | Infiltrative, can show vascular invasion and necrosis |
| Immunohistochemistry | Epithelial cells negative for c-KIT (CD117). Immature TDT-positive T-cells present | Epithelial cells positive for c-KIT (CD117) in 60%-80% of cases. CD5 often expressed |
| Imaging | More regular, less necrotic, and cystic | Irregular borders, necrotic and cystic areas Lymphadenopathy and/or, pleural effusion |
Gross appearance
On gross examination, TC appears large, firm, infiltrating, and can have cystic changes or necrosis. Up to 15% of TC can be encapsulated[17]. Some gross examination clues can suggest a particular histological subtype, such as the appearance of a mucoid cut surface, which may hint toward a mucoepidermoid carcinoma[18].
RESULTS
Histological subtypes
In 1982, Snover et al.[19] originally defined five distinct histologic variants of TC. The subtypes are similar to carcinomas seen in other organs and indicate that the thymic epithelium can differentiate in many ways.
In 2021, the fifth edition of the World Health Organization (WHO) Classification of Thoracic Tumors was released with the recognized categories of TC largely remaining the same when compared to the fourth edition[20]. The primary changes in the WHO 2021 include the addition of a hyalinizing subtype characterized by an EWSR1 translocation for clear cell carcinoma, which corresponds to the same hyalinizing subtype of clear cell carcinoma of the salivary gland. Another change is in the terminology of adenocarcinomas, where “mucinous adenocarcinoma” is changed to “enteric-type adenocarcinoma”, and “papillary adenocarcinoma” is changed to “low-grade papillary adenocarcinoma” due to its bland histologic features and rather indolent clinical course. Lastly, “micronodular thymic carcinoma with lymphoid hyperplasia” as a subtype of squamous cell carcinoma was added.
Combined TC with more than one histological type is not common, but is reported and can occur. If present, the features of the different components should be reported in 10% increments and termed “combined thymic carcinoma”, which is in line with the WHO 2021 recommendation.
Squamous cell carcinoma of the thymus
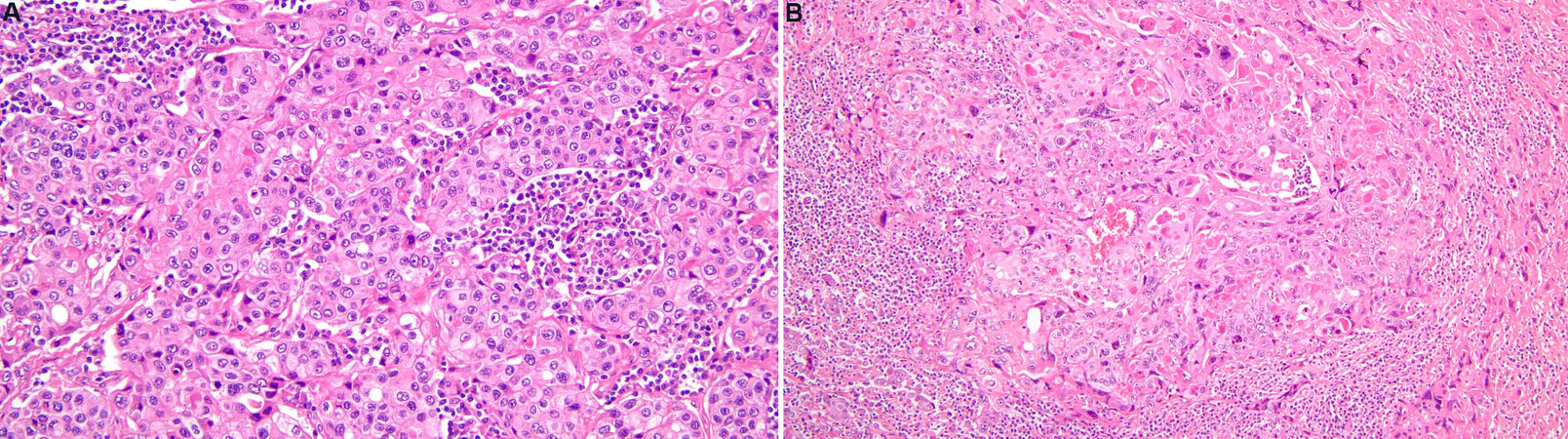
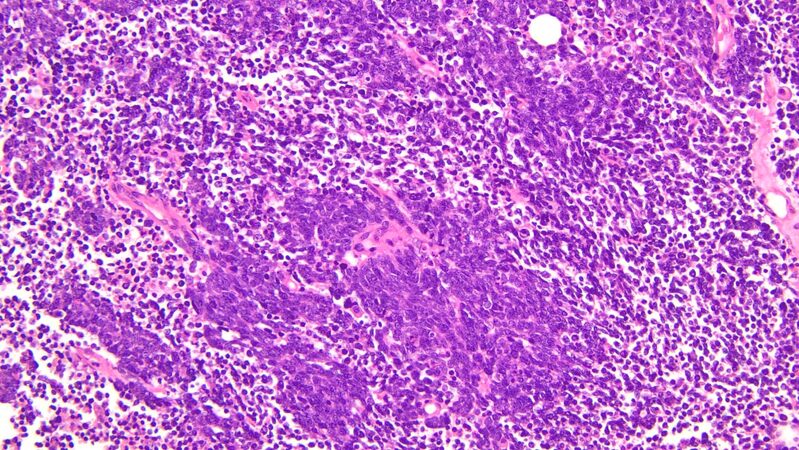
Squamous cell carcinoma of the thymus is the most common morphological subtype accounting for approximately 70% of all thymic carcinomas[21,22]. Most commonly, it occurs in the sixth decade with a slight male predominance. Clinically, it often presents with symptoms associated with mass effect within the mediastinum. Up to a third of the patients are diagnosed incidentally on imaging[23]. On imaging, the lesions present as more irregular with necrotic and cystic components. The majority of patients present at advanced clinical stages. Histologically, this entity is comparable to squamous cell carcinomas arising in other locations, and it is important to exclude metastasis from other organs. Squamous cell carcinoma does not resemble normal thymic architecture, can present as keratinizing or non-keratinizing, lacks encapsulation, and can grow in nests with a round to oval nuclei [Figure 1A and B]. EGFR mutations are potentially associated with a worse prognosis[24].
Figure 1. Thymic squamous cell carcinoma. Different varieties of thymic squamous cell carcinoma, including non-keratinizing (A, 20×) and keratinizing (B, 10×) forms.
On immunostaining, the tumor cells are positive for pankeratin. CD5, c-KIT (CD117), GLUT1, and MUC1 can also be positive. p16 expression is found in approximately 65% of thymic squamous cell carcinomas[25].
In the recent WHO, micronodular thymic carcinoma with lymphoid hyperplasia was added as a subtype of squamous cell carcinoma, which is the malignant counterpart to micronodular thymoma with lymphoid hyperplasia[26]. This carcinoma shows lymphoid stroma with prominent germinal centers and nodules of spindle cells, which show marked atypia. Compared to micronodular thymoma, micronodular thymic carcinoma with lymphoid hyperplasia lacks TdT-positive T-cells around the tumor islands and the tumor cells often express c-KIT (CD117) and CD5.
Basaloid carcinoma of the thymus
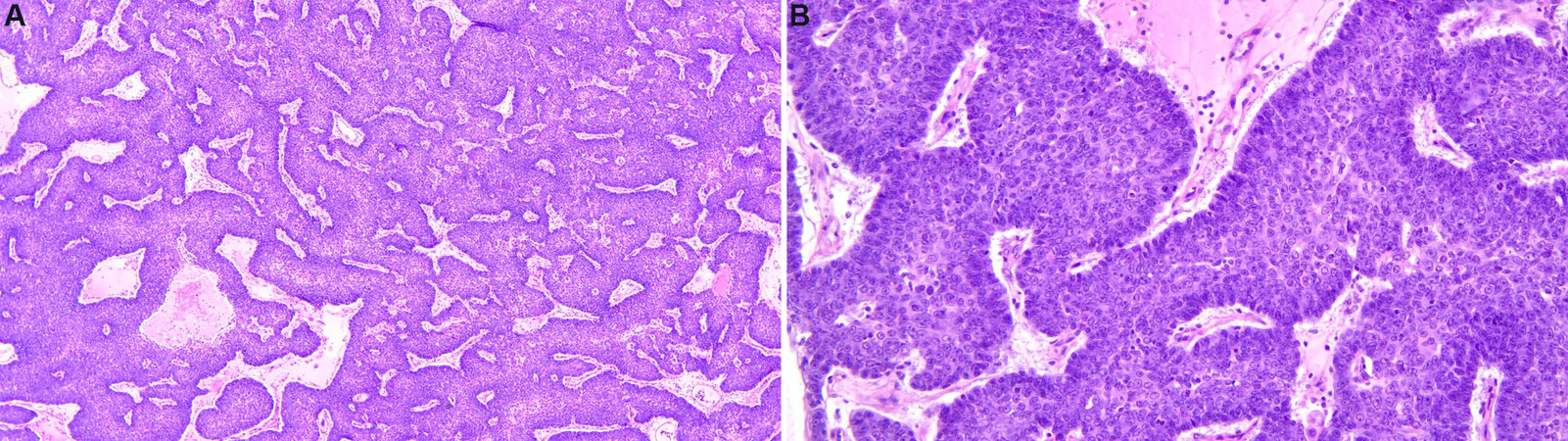
Basaloid carcinoma is a rare subtype of TC and represents less than 5% of all TC. It occurs most commonly between the fifth and sixth decade and has a slight male predominance. About 60% of the cases are diagnosed incidentally on imaging. Histologically, basaloid carcinoma of the thymus presents itself with small nests/cords of hyperchromatic round to oval tumor cells that show prominent peripheral palisading of nuclei with a subset demonstrating cystic changes [Figure 2A and B]. Basaloid carcinomas are typically associated with a less aggressive clinical course. On immunostaining, the tumor cells are positive for pankeratin. Approximately 75% of cases are positive for p40/p63 and c-KIT (CD117)[27].
Mucoepidermoid carcinoma of the thymus
Mucoepidermoid carcinoma is a rare subtype of TC, accounting for less than 5% of all TC. Histologically, it presents as a mix of squamous and glandular components[28]. Intermediate cells with polygonal shapes and eosinophilic cytoplasm can be observed. The squamous epithelial aspect of this carcinoma usually presents as sheet-like growth with atypical cells that display keratinization and intercellular bridges
Lymphoepithelial carcinoma of the thymus
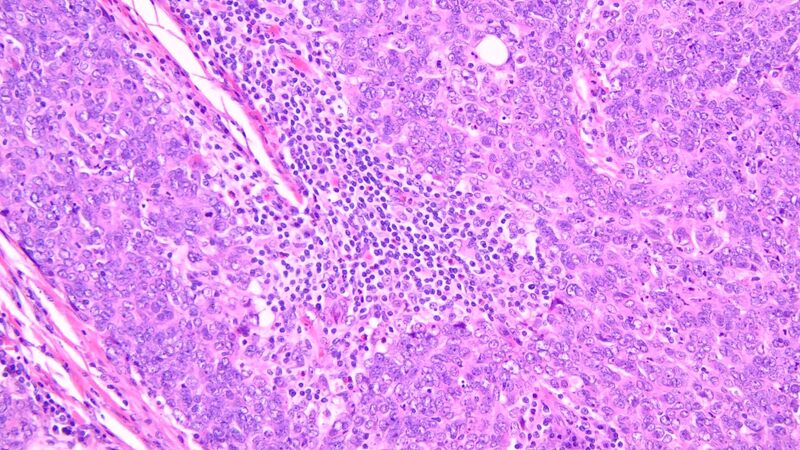
Lymphoepithelial carcinoma, formerly known as lymphoepithelioma-like carcinoma, is a rare TC accounting for about 1.3%-6% of all TC[29]. Although the term of lymphoepithelial carcinoma denotes a prominent lymphoid background, these tumors could be conceptualized as poorly-differentiated, non-keratinizing squamous cell carcinomas in their appearance. An association with Epstein-Barr virus (EBV) in the pathogenesis of this tumor has been reported[30-32]. Histologically, the tumor presents as a poorly differentiated, nonkeratinizing squamous cell carcinoma. The carcinoma has large, polygonal cells and shows a syncytial growth pattern. A prominent lymphoid background with scattered plasma cells is seen surrounding the tumor cells [Figure 4]. By immunohistochemistry, tumor cells show variable reactivity for CD5, are positive for c-KIT (CD117), and commonly express PD-L1[33]. If associated with EBV, the tumor cells show positive nuclear labeling for EBV-encoded small RNA on in-situ hybridization. The inflammatory component is highlighted by mature B- and T-cells with polyclonal plasma cells.
Sarcomatoid carcinoma of the thymus
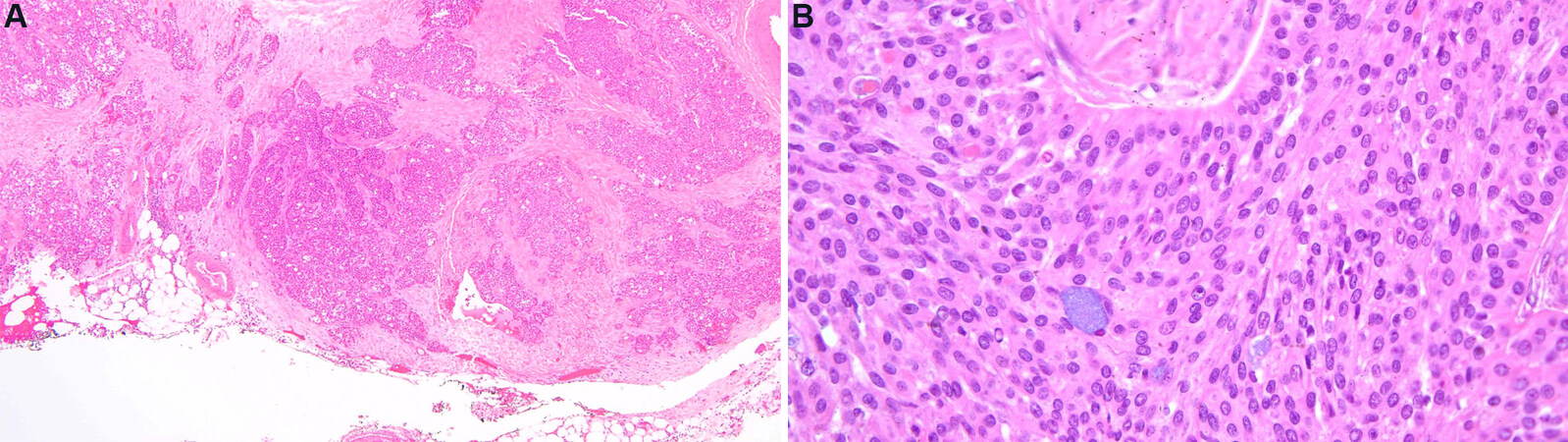
Sarcomatoid carcinomas account for approximately 5% of all TC. Clinically, these carcinomas present with similar symptoms as other TC and show very aggressive behavior with the majority of patients presenting with advanced disease at diagnosis. A comprehensive case series by Suster and Moran[34] described the microscopic features of this tumor as showing a spindle cell proliferation with a high mitotic count [Figure 5]. In a minority of instances, the tumor can appear similar to a spindle cell thymoma. Some areas can show a different growth pattern, such as rhabdomyoblastic or cartilaginous differentiation. Sarcomatoid carcinoma arising from metaplastic thymoma has been described[35,36].
Clear cell carcinoma of the thymus
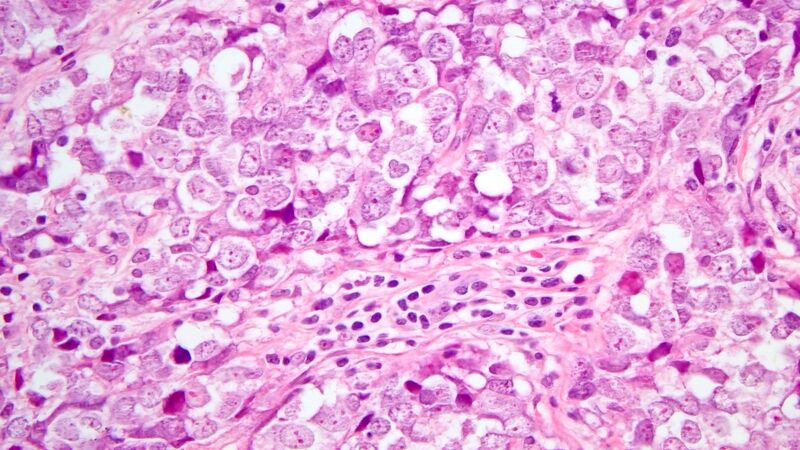
Originally described by Snover et al.[19], this TC shows a lobulated growth pattern with cellular morphology ranging from uniform clear cells with minimal nuclear pleomorphism to larger, more pleomorphic tumor cells with prominent nucleoli [Figure 6]. There is no glandular component, and lymphocytes can be admixed within the tumor. Cells show abundant cytoplasmic glycogen that is Periodic acid-Schiff (PAS) positive and is usually negative for mucicarmine. Clinically, these carcinomas have a poor prognosis. A large case series by Hasserjian et al.[37] described the clinical symptoms, which are similar to other TC with overall survival estimated at 4-24 months.
Figure 6. Clear cell carcinoma (40×). A dense infiltrate of large, pleomorphic cells with intracytoplasmic glycogen that is Periodic acid-Schiff (PAS) positive.
Cases of thymic carcinoma with clear cell morphology and substantial hyalinization carrying an EWSR translocation have been described[38]. In the most recent WHO classification, hyalinizing clear cell carcinoma with EWSR1 translocation is recognized as a potential subtype of clear cell carcinoma.
Low-grade papillary adenocarcinoma of the thymus
Low-grade papillary adenocarcinoma of the thymus is reported to occur in up to 3% of TC. An association between low-grade papillary adenocarcinomas of the thymus and type A thymoma, A/B thymoma, or multilocular thymic cysts have been made[39]. Clinically, an elevated carcinoembryonic antigen level has been reported[40]. Histologically, this tumor shows predominantly tubular and papillary structures with fibrovascular cores that are separated by fibroconnective tissue. Psammoma bodies can be present. No prominent nuclear atypia is seen. If high-grade atypia is present, a diagnosis of thymic adenocarcinoma, NOS should be considered. Immunohistochemistry can be helpful in excluding papillary neoplasms of thyroid, lung, and other extrathoracic sites, as the tumor cells are negative for thyroglobulin, TTF-1, calcitonin, CK20, CDX2, CD20, and PAX8[41].
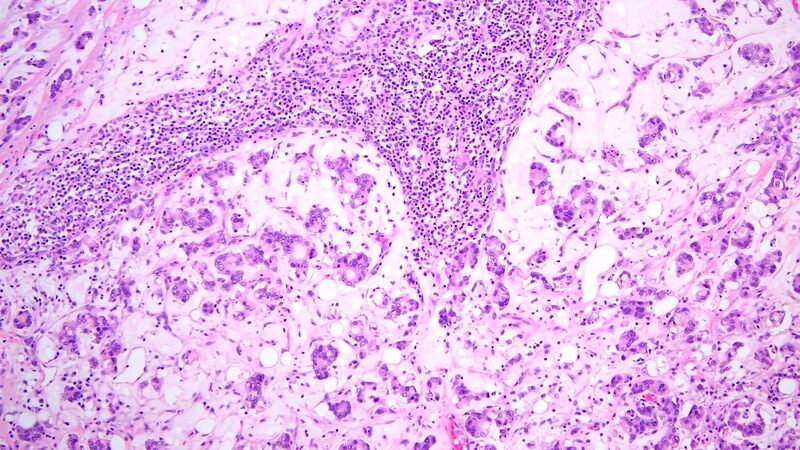
Enteric-type adenocarcinoma of the thymus
Enteric-type adenocarcinoma of the thymus accounts for less than 5% of all TC, and is more commonly seen in male patients. The term “mucinous (colloid) adenocarcinoma” is no longer recommended as the WHO preferred nomenclature is “enteric-type adenocarcinoma of the thymus”. Previously, mucinous adenocarcinoma has been divided into enteric, mucinous, and colloid types, which indicate the different types of mucin[42]. Histologically, enteric-type adenocarcinoma is composed of small cells present within extracellular mucin [Figure 7]. By immunohistochemistry, tumor cells are positive for at least one marker of the gastrointestinal tract (CK20, CDX2, and/or MUC2)[43]. Tumor cells are negative for TTF-1 and c-KIT (CD117)[44]. KRAS, TP53, TGFB2 mutations commonly seen in adenocarcinomas of the gastrointestinal tract have been described in enteric-type adenocarcinoma of the thymus[45,46].
Adenocarcinoma, not otherwise specified
Adenocarcinoma, not otherwise specified (NOS) encompasses TC not meeting the diagnostic criteria for low-grade papillary or enteric-type adenocarcinoma[47]. The recent WHO classification includes the following provisional categories: tubular adenocarcinoma, non-mucinous adenocarcinoma, high-grade papillary adenocarcinoma, and signet-ring cell carcinoma with a gastric immunophenotype. By immunohistochemistry, the carcinomas of this category do not express gastrointestinal markers, are positive for epithelial markers, and show variable expression profiles for c-KIT (CD117) and CD5.
Adenosquamous carcinoma
Adenosquamous carcinoma, similar to its pulmonary counterpart, is a primary TC that shows both glandular and squamous differentiation, where each component constitutes ≥ 10% of the tumor. If the glandular component is < 10%, a diagnosis of squamous cell carcinoma is preferred. Only rare case reports are available describing adenosquamous carcinomas[48]. By immunohistochemistry, the tumor is positive for epithelial markers, and the squamous component is highlighted by CK5/6 and p63/p40.
NUT carcinoma of the thorax
NUT carcinoma of the thorax is defined by the presence of the NUTM1 gene rearrangement. These carcinomas behave aggresively and are most commonly diagnosed at an advanced disease stage with a reported median survival of 6.5 months[49]. Histologically, they present as tumors with sheets and nests of monotonous, small- to intermediate-sized, undifferentiated round to oval cells with prominent nucleoli. The cells are evenly spaced with a distinct separation between the cells. Foci of abrupt keratinization are estimated to be present in about one-third of the cases. A glandular component is not present. NUT immunostain reveals a speckled nuclear expression pattern in about 87% of the cases[50]. Tumor cells can stain positive for CD34, which could represent a potential diagnostic pitfall[51]. Together with the NUT immunostain, identifying NUTM1 rearrangement by molecular methods plays a key role in diagnosing this TC subtype. The most common translocation is t(15;19)(q14;p13.1), resulting in a fusion of NUTM1 and BRD4[52]. Less commonly, fusions of NUTM1 to other genes encoding for proteins related to BRD4 have been reported (BRD3, NSD3, ZNF532, ZNF592)[53-55].
Thymic carcinoma with adenoid cystic carcinoma-like features
Thymic carcinoma with adenoid cystic carcinoma-like features is a rare TC subtype with only a few cases reported in the literature. Cases are described as havingindolent behavior[56,57]. Histologically, the tumor shows features comparable to adenoid cystic carcinoma of the salivary glands and is made up of basaloid cells growing in a cribriform pattern. The tumor cells are small to intermediate in size with mild to moderate nuclear atypia. Cystic structures are seen with spaces filled with cellular debris and PAS-negative material. The material within the cysts is positive for basement membrane markers, such as laminin or collagen IV. Compared to adenoid cystic carcinoma, thymic carcinoma with adenoid cystic carcinoma-like features is negative for c-KIT (CD117), S-100, and smooth muscle markers[58].
Undifferentiated carcinoma of the thymus
The category of undifferentiated carcinoma of the thymus represents a group of TC that are poorly differentiated and not assignable to other histologic subtypes identified by the recent WHO classification. Marked cellular pleomorphism and cellular atypia are present with high mitotic activity. Necrosis and hemorrhage are frequently present. Clinically, the tumors are highly aggressive and have poor outcomes.
Thymic carcinoma, NOS
Thymic carcinoma, NOS is a unique category of TC in which the carcinoma cannot be further categorized under WHO-defined entities. Table 2 highlights exceedingly rare variants of TC and their features.
Thymic carcinoma, NOS subtypes
| Type | Features |
| Rhabdoid thymic carcinoma | TC showing a solid proliferation of large, pleomorphic cells with large nuclei, prominent nucleoli, and arranged in nests and sheets[59,60] |
| Hepatoid thymic carcinoma | Morphologic features resemble hepatocellular carcinoma, and by immunohistochemistry, are positive for hepatocyte paraffin 1[61,62] |
| Undifferentiated large cell carcinoma associated with Castleman disease-like reaction | TC displaying solid nests of undifferentiated, large cells in a background of lymphoid stroma that resembles hyaline-vascular Castleman disease and often display a less aggressive clinical course[63] |
DISCUSSION
TC is a heterogeneous group of rare primary TEN that are clinically aggressive, often present at an advanced stage, and have characteristic molecular or cytogenetics findings in only a subset of cases. As TC is part of the differential of invasive anterior mediastinal masses that present with local symptoms, a core biopsy approach is often undertaken; however, this can provide a diagnostic challenge. Certain clues can help in the correct interpretation of core biopsies, such as marked atypia, frequent mitotic figures, infiltration, necrosis, and expression of c-KIT (CD117); however, interpretation may remain a challenge when metastatic tumors are within the differential diagnosis. If the initial core biopsy remains equivocal, thoracoscopic biopsies are useful in that adequate tissue is often obtained while also giving the advantage of visualizing the hemithorax for staging purposes and the ability to sample lymph nodes and pleura.
As a refinement of TC knowledge has changed over the years, there remains a fundamental conundrum when evaluating the histopathology of these tumors. Recent changes to the WHO have revealed only minor alterations in terminology, but now recognize the reporting of heterogeneous tumors. It is common for thymomas to harbor more than one histologic pattern; however, reporting this phenomenon in TC is relatively scarce. With this in mind, tumor heterogeneity may be misrepresented on core biopsies, thus limiting findings that are of diagnostic and prognostic significance. It is therefore important to correlate histologic and immunophenotypic findings with that of radiographic imaging; however, more studies are needed as to what degree of heterogeneity and morphologic components contribute to the outcome.
In the era of molecular diagnostics, future analyses of thymic carcinoma will prove to be a fruitful endeavor, given the rarity and aggressiveness of these tumors. It remains hopeful that the utilization or development of targeted therapies will change the clinical outcomes for patients, such as in the case of squamous cell carcinoma of the thymus harboring an activating KIT mutation and its responsiveness to imatinib[64]. Additionally, the utilization of next-generation sequencing has provided an insight into the molecular pathogenesis of thymic carcinoma, which can be further utilized to provide prognostic information for these tumors.
Lastly, the most appropriate staging schema for thymic carcinoma has long been fraught with controversy. It is recognized that certain staging methods lack definitive differentiation between thymoma and thymic carcinoma, particularly in regard to capsular invasion, which carries significantly different prognostic features depending on the final diagnosis. In the recent National Comprehensive Cancer Network Clinical Practical Guidelines in Oncology for thymomas and thymic carcinoma, encapsulated thymomas and stage I thymic carcinoma are effectively labeled as equal in staging, which would portend a similar clinical course[65]. This lack of uniformity in staging and applications in practice underscores the need for a systematic staging methodology founded on sound data and the understanding of disease biology.
The landscape of TC has changed considerably since the first description of TEN, particularly in regard to terminology, immunoprofile, and now with the advent of molecular diagnostic techniques, its underlying pathobiology. It will be of great interest to many pathologists, oncologists, surgeons, and radiologists to stay tuned for changes on the horizon as there still remains much to be discovered within the world of TC.
DECLARATIONS
Authors’ contributionsKarlin Wrote the manuscript: Karlin K
Reviewed and edited the manuscript: Michaels PD
All authors approved the manuscript.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestBoth authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Ahmad U, Yao X, Detterbeck F, et al. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg 2015;149:95-100, 101.e1.
2. Chung DA. Thymic carcinoma--analysis of nineteen clinicopathological studies. Thorac Cardiovasc Surg 2000;48:114-9.
3. Eng TY, Fuller CD, Jagirdar J, Bains Y, Thomas CR Jr. Thymic carcinoma: state of the art review. Int J Radiat Oncol Biol Phys 2004;59:654-64.
4. Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884.
5. Quagliano PV. Thymic carcinoma: case reports and review. J Thorac Imaging 1996;11:66-74.
6. Nasseri F, Eftekhari F. Clinical and radiologic review of the normal and abnormal thymus: pearls and pitfalls. Radiographics 2010;30:413-28.
7. Roden AC, Erickson-Johnson MR, Yi ES, García JJ. Analysis of MAML2 rearrangement in mucoepidermoid carcinoma of the thymus. Hum Pathol 2013;44:2799-805.
8. Zettl A, Ströbel P, Wagner K, et al. Recurrent genetic aberrations in thymoma and thymic carcinoma. Am J Pathol 2000;157:257-66.
9. Radovich M, Pickering CR, Felau I, et al. The integrated genomic landscape of thymic epithelial tumors. Cancer Cell 2018;33:244-58.e10.
10. Suster S, Rosai J. Thymic carcinoma. A clinicopathologic study of 60 cases. Cancer 1991;67:1025-32.
11. Suster S, Moran CA. Primary thymic epithelial neoplasms showing combined features of thymoma and thymic carcinoma. A clinicopathologic study of 22 cases. Am J Surg Pathol 1996;20:1469-80.
13. Weissferdt A, Moran CA. Immunohistochemistry in the diagnosis of thymic epithelial neoplasms. Appl Immunohistochem Mol Morphol 2014;22:479-87.
14. Nakagawa K, Matsuno Y, Kunitoh H, Maeshima A, Asamura H, Tsuchiya R. Immunohistochemical KIT (CD117) expression in thymic epithelial tumors. Chest 2005;128:140-4.
15. Pan CC, Chen PC, Chiang H. KIT (CD117) is frequently overexpressed in thymic carcinomas but is absent in thymomas. J Pathol 2004;202:375-81.
16. Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization Classification of tumors of the thymus: continuity and changes. J Thorac Oncol 2015;10:1383-95.
17. Truong LD, Mody DR, Cagle PT, Jackson-York GL, Schwartz MR, Wheeler TM. Thymic carcinoma. A clinicopathologic study of 13 cases. Am J Surg Pathol 1990;14:151-66.
18. Moran CA, Suster S. Mucoepidermoid carcinomas of the thymus. A clinicopathologic study of six cases. Am J Surg Pathol 1995;19:826-34.
19. Snover DC, Levine GD, Rosai J. Thymic carcinoma. Five distinctive histological variants. Am J Surg Pathol 1982;6:451-70.
20. . WHO Classification of Tumours Editorial Board. Thoracic tumours. 5th ed. Lyon, France: International Agency for Research on Cancer; 2021
21. Weissferdt A, Moran CA. Thymic carcinoma, part 1: a clinicopathologic and immunohistochemical study of 65 cases. Am J Clin Pathol 2012;138:103-14.
22. Weksler B, Dhupar R, Parikh V, Nason KS, Pennathur A, Ferson PF. Thymic carcinoma: a multivariate analysis of factors predictive of survival in 290 patients. Ann Thorac Surg 2013;95:299-303.
23. Zhao Y, Zhao H, Hu D, Fan L, Shi J, Fang W. Surgical treatment and prognosis of thymic squamous cell carcinoma: a retrospective analysis of 105 cases. Ann Thorac Surg 2013;96:1019-24.
24. Girard N, Shen R, Guo T, et al. Comprehensive genomic analysis reveals clinically relevant molecular distinctions between thymic carcinomas and thymomas. Clin Cancer Res 2009;15:6790-9.
25. Suster D, Pihan G, Mackinnon AC, Suster S. Poorly differentiated nonkeratinizing squamous cell carcinoma of the thymus: clinicopathologic and molecular genetic study of 25 cases. Am J Surg Pathol 2018;42:1224-36.
26. Thomas de Montpreville V, Mansuet-Lupo A, Le Naoures C, et al. Micronodular thymic carcinoma with lymphoid hyperplasia: relevance of immunohistochemistry with a small panel of antibodies for diagnosis-a RYTHMIC study. Virchows Arch 2021;479:741-6.
27. Brown JG, Familiari U, Papotti M, Rosai J. Thymic basaloid carcinoma: a clinicopathologic study of 12 cases, with a general discussion of basaloid carcinoma and its relationship with adenoid cystic carcinoma. Am J Surg Pathol 2009;33:1113-24.
28. Chalabreysse L, Etienne-Mastroianni B, Adeleine P, Cordier JF, Greenland T, Thivolet-Bejui F. Thymic carcinoma: a clinicopathological and immunohistological study of 19 cases. Histopathology 2004;44:367-74.
29. Hishida T, Nomura S, Yano M, et al. Japanese Association for Research on the Thymus (JART). Long-term outcome and prognostic factors of surgically treated thymic carcinoma: results of 306 cases from a Japanese Nationwide Database Study. Eur J Cardiothorac Surg 2016;49:835-41.
30. Leyvraz S, Henle W, Chahinian AP, et al. Association of Epstein-Barr virus with thymic carcinoma. N Engl J Med 1985;312:1296-9.
31. Xu M, Yao Y, Chen H, et al. Genome sequencing analysis identifies Epstein-Barr virus subtypes associated with high risk of nasopharyngeal carcinoma. Nat Genet 2019;51:1131-6.
32. Zhang G, Yu Z, Shen G, Chai Y, Liang C. Association between Epstein-Barr virus and Thymic epithelial tumors: a systematic review. Infect Agent Cancer 2019;14:32.
33. Suster D, Pihan G, Mackinnon AC, Suster S. Expression of PD-L1/PD-1 in lymphoepithelioma-like carcinoma of the thymus. Mod Pathol 2018;31:1801-6.
34. Suster S, Moran CA. Spindle cell thymic carcinoma: clinicopathologic and immunohistochemical study of a distinctive variant of primary thymic epithelial neoplasm. Am J Surg Pathol 1999;23:691-700.
35. Moritani S, Ichihara S, Mukai K, et al. Sarcomatoid carcinoma of the thymus arising in metaplastic thymoma. Histopathology 2008;52:409-11.
36. Lu HS, Gan MF, Zhou T, Wang SZ. Sarcomatoid thymic carcinoma arising in metaplastic thymoma: a case report. Int J Surg Pathol 2011;19:677-80.
37. Hasserjian RP, Klimstra DS, Rosai J. Carcinoma of the thymus with clear-cell features. Report of eight cases and review of the literature. Am J Surg Pathol 1995;19:835-41.
38. Porubsky S, Rudolph B, Rückert JC, et al. International Thymic Malignancy Interest Group (ITMIG). EWSR1 translocation in primary hyalinising clear cell carcinoma of the thymus. Histopathology 2019;75:431-6.
39. Matsuno Y, Morozumi N, Hirohashi S, Shimosato Y, Rosai J. Papillary carcinoma of the thymus: report of four cases of a new microscopic type of thymic carcinoma. Am. J Surg Pathol 1998;22:873-80.
40. Oka S, Inoue M, Honda Y, et al. Thymic papillary adenocarcinoma coexisting with type A thymoma: a case report. Int J Surg Case Rep 2019;57:142-44.
41. Kalhor N, Moran CA. Primary thymic adenocarcinomas: a clinicopathological and immunohistochemical study of 16 cases with emphasis on the morphological spectrum of differentiation. Hum Pathol 2018;74:73-82.
42. Moser B, Schiefer AI, Janik S, et al. Adenocarcinoma of the thymus, enteric type: report of 2 cases, and proposal for a novel subtype of thymic carcinoma. Am J Surg Pathol 2015;39:541-8.
43. Kwon AY, Han J, Chu J, et al. Histologic characteristics of thymic adenocarcinomas: clinicopathologic study of a nine-case series and a review of the literature. Pathol Res Pract 2017;213:106-12.
44. Maeda D, Ota S, Ikeda S, et al. Mucinous adenocarcinoma of the thymus: a distinct variant of thymic carcinoma. Lung Cancer 2009;64:22-7.
45. Sakanoue I, Hamakawa H, Fujimoto D, et al. KRAS mutation-positive mucinous adenocarcinoma originating in the thymus. J Thorac Dis 2017;9:E694-7.
46. Lee Y, Park S, Lee SH, Lee H. Characterization of genetic aberrations in a single case of metastatic thymic adenocarcinoma. BMC Cancer 2017;17:330.
47. Maghbool M, Ramzi M, Nagel I, et al. Primary adenocarcinoma of the thymus: an immunohistochemical and molecular study with review of the literature. BMC Clin Pathol 2013;13:17.
48. Takahashi K, Yoshida J, Nishimura M, Nagai K. Thymic carcinoma. Outcome of treatment including surgical resection. Jpn J Thorac Cardiovasc Surg 2000;48:494-8.
49. Chau NG, Ma C, Danga K, et al. An anatomical site and genetic-based prognostic model for patients with nuclear protein in testis (NUT) midline carcinoma: analysis of 124 patients. JNCI Cancer Spectr 2019;4:pkz094.
50. Haack H, Johnson LA, Fry CJ, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol 2009;33:984-91.
51. French CA, Kutok JL, Faquin WC, et al. Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol 2004;22:4135-9.
52. Gökmen-Polar Y, Cano OD, Kesler KA, Loehrer PJ, Badve S. NUT midline carcinomas in the thymic region. Mod Pathol 2014;27:1649-56.
53. French CA, Miyoshi I, Kubonishi I, Grier HE, Perez-Atayde AR, Fletcher JA. BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res 2003;63:304-7.
54. French CA, Rahman S, Walsh EM, et al. NSD3-NUT fusion oncoprotein in NUT midline carcinoma: implications for a novel oncogenic mechanism. Cancer Discov 2014;4:928-41.
55. Shiota H, Elya JE, Alekseyenko AA, et al. “Z4” complex member fusions in NUT carcinoma: implications for a novel oncogenic mechanism. Mol Cancer Res 2018;16:1826-33.
56. Banki F, Khalil K, Kott MM, Cota AL. Adenoid cystic carcinoma of the thymus gland: a rare tumor. Ann Thorac Surg 2010;90:e56-8.
57. Yang MQ, Bai LL, Wang Z, Huang WJ, Jiang GY, Xu HT. Primary thymic carcinoma with adenoid cystic carcinoma-like features: a case report and literature review. Medicine (Baltimore) 2020;99:e21531.
58. Kanzaki R, Ikeda N, Okura E, et al. Thymic carcinoma with adenoid cystic carcinomalike features with distant metastases. Ann Thorac Cardiovasc Surg 2012;18:544-7.
59. Toprani TH, Tamboli P, Amin MB, Ordoñez NG, Ayala AG, Ro JY. Thymic carcinoma with rhabdoid features. Ann Diagn Pathol 2003;7:106-11.
60. Maralakunte M, Singh L, Debi U, Karki T, Aggarwal D, Bal A. Malignant rhabdoid tumor of the mediastinum: a rare variant of thymic carcinoma presenting as superior vena caval syndrome. Arch Clin Med Case Rep 2020;4:717-23.
61. Franke A, Ströbel P, Fackeldey V, et al. Hepatoid thymic carcinoma: report of a case. Am J Surg Pathol 2004;28:250-6.
62. Jeong JS, Kang HJ, Jo U, Song MJ, Nam SY, Song JS. Hepatoid thymic carcinoma: a case report of a rare subtype of thymic carcinoma. J Pathol Transl Med 2021;55:230-4.
63. Nonaka D, Rodriguez J, Rollo JL, Rosai J. Undifferentiated large cell carcinoma of the thymus associated with Castleman disease-like reaction: a distinctive type of thymic neoplasm characterized by an indolent behavior. Am J Surg Pathol 2005;29:490-5.
64. Ströbel P, Hartmann M, Jakob A, et al. Thymic carcinoma with overexpression of mutated KIT and the response to imatinib. N Engl J Med 2004;350:2625-6.
65. National Comprehensive Cancer network. NCCN clinical practice guidelines in oncology (NCCN guidelines) Thymoma and Thymic carcinoma. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx [Last accessed on 24 Mar 2022].
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Karlin K, Michaels PD. Thymic carcinoma: review and update. J Cancer Metastasis Treat 2022;8:15. http://dx.doi.org/10.20517/2394-4722.2021.185
AMA Style
Karlin K, Michaels PD. Thymic carcinoma: review and update. Journal of Cancer Metastasis and Treatment. 2022; 8: 15. http://dx.doi.org/10.20517/2394-4722.2021.185
Chicago/Turabian Style
Karlin, Kirill, Phillip D. Michaels. 2022. "Thymic carcinoma: review and update" Journal of Cancer Metastasis and Treatment. 8: 15. http://dx.doi.org/10.20517/2394-4722.2021.185
ACS Style
Karlin, K.; Michaels PD. Thymic carcinoma: review and update. J. Cancer. Metastasis. Treat. 2022, 8, 15. http://dx.doi.org/10.20517/2394-4722.2021.185
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 25 clicks
Cite This Article 25 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.