Surgical route and pathological risk factors in early cervical cancer - Node Zero (SURPEC-N0)
Abstract
Aim: The aim of this study is to compare disease-free survival (DFS) and overall survival (OS) in patients with stage I cervical cancer (≤ 4cms, lymph node-negative) undergoing open radical hysterectomy (ORH) vs. minimally invasive radical hysterectomy (MIRH).
Methods: All patients undergoing radical hysterectomy between January 2012-December 2018 from the largest tertiary referral cancer centre were included. A 1:1 propensity matching was done based on four independent prognostic factors to compare DFS and OS with the route of surgery.
Results: One hundred and ninety-nine patients were included during the study period. The median age of the cohort was 50 years. The median follow-up of patients was 47 months. Following 1:1 propensity matching, a total of 174 patients were analysed for DFS and OS in ORH (n = 87) and MIRH (n = 87) groups. Protective measure was used in two-thirds of the patients during MIRH. Twenty-nine patients (16.7%) had recurrences. For the matched cohort (n = 174), the DFS at 36 and 60 months was 84.8% (78.1%-89.6%) and 81% (73.4%-86.6%) respectively and the OS was 96.5% (91.7%-98.5%) and 95.6% (90.3%-98%) respectively. There was no statistically significant difference in DFS or OS between ORH and MIRH.
Conclusion: The present study showed no difference in oncological outcomes in MIRH compared to ORH. Retrospective audits on patient characteristics such as screening/vaccination history along with surgical technique/load and matching for crucial risk factors should be factored in future studies to eliminate the possible methodological errors.
Keywords
INTRODUCTION
Open radical hysterectomy (ORH) for operable invasive cervical cancer has undergone various modifications for over a century before being accepted as a standard surgical procedure. Minimally invasive radical hysterectomy (MIRH) has been increasingly performed over the last two decades and was established as the preferred surgical modality for treating early cervical cancer based on the demonstration of equivalent survival figures and better surgical outcome compared to the open approach[1]. Many publications in literature showed the feasibility, safety and advantages with MIRH, such as less postoperative pain, lower incidence of postoperative complications and faster recovery compared to the open approach[2-5].
However, the results of the minimal access approach to cervical cancer (LACC) trial published in November 2018 in NEJM had suggested adverse oncological outcomes with minimal access route compared to open route[6]. Since then, various published retrospective data and meta-analyses have demonstrated both adverse or no difference in oncological outcomes with MIRH in comparison to ORH[7-13]. The majority of cervical cancers analysed in the above-mentioned published studies are in the screened population. Despite the reduction in incidence rates of invasive cervical cancer in India in various urban and rural registries, the mortality from cervical cancer has not reduced in linear fashion accordingly[14]. The natural history of disease, tumour biology and oncological outcomes with respect to the route of surgery in an unscreened population is unknown and not comparable.
Hence, we designed a retrospective study of patients who developed cervical cancer in an unscreened population and underwent radical hysterectomy for stage I (≤ 4 cm) cervical cancer at a large tertiary cancer centre.
The primary and secondary objective of the study is to compare disease-free survival and overall survival, respectively, in patients with stage I (≤ 4cms) cervical cancer undergoing ORH and MIRH.
METHODS
Propensity matched analysis of patients with a diagnosis of early cervical cancer (stage I ≤ 4cms) undergoing radical hysterectomy between January 2012-December 2018 at a single tertiary referral cancer centre was performed. Demographic and disease characteristics, treatment, and follow up details were gathered from hospital electronic records.
The inclusion criteria were defined as patients undergoing radical hysterectomy and pelvic lymphadenectomy, age between 18-75 years, clinical/histopathology showing ≤ 4cm cervical cancer with squamous or adenocarcinoma histology. Patients having tumour size > 4cms either clinically or on surgical pathology, lymph-node positive disease, those who received neoadjuvant chemotherapy, chemoradiation or radiation before surgery and histology other than squamous or adenocarcinoma were excluded.
As per hospital protocol, radical hysterectomy was abandoned if pelvic lymph nodes were positive for tumour on frozen section. Postoperatively based on final histopathology reports, patients were stratified into low, intermediate and high risk based on standard international criteria[15,16]. High-risk category was defined as either lymph node-positive disease or positive parametrium or positive margins on final histology. Intermediate risk category was considered when any two of these features were present- stromal invasion ≥ 50%, tumour size > 4 cm and lymphovascular space invasion.
Adjuvant treatment was given after discussion in multi-disciplinary team clinics according to the risk stratification. Standard template external beam pelvic radiation along with vaginal brachytherapy was given for intermediate-risk factors, and concurrent chemoradiation with weekly cisplatin was given for high-risk factors. Patients were then followed up as per hospital protocol every 3-6 months for a minimum of 5-10 years. Recurrence was confirmed by a combination of clinical, radiological or histopathology findings. Disease-free survival (DFS) was defined as the time from the date of completion of primary treatment to the time of relapse or last contact. Overall survival was calculated from the date of completion of primary treatment to the time of last contact or death from cervical cancer. Kaplan-Meier method was used for the estimation of the probability of disease-free survival (DFS) and overall survival (OS). Four factors (tumour size, histological type, postoperative risk stratification, postoperative adjuvant treatment) which have a direct effect on DFS and OS were considered in propensity matching. Propensity matching was performed prior to comparing DFS and OS in the study population.
Statistical analysis
The categorical variables were expressed as frequencies and percentages; the continuous variables were expressed as means and SDs. The analysis of associations between open and MIRH group for categorical data was performed using a Pearson’s Chi-square test or Fisher exact test in cases with small counts. Significance of time-dependent outcomes, including DFS and OS, was examined with the log-rank test. Cox’s proportional hazard regression test was used to calculate the hazard ratio. Survival curves were constructed with the Kaplan-Meier method. P-values of less than 0.05 were considered statistically significant (2-tailed).
The matching on the propensity score (1:1) was performed using an exact matching algorithm. Match adequacy was determined using standardised differences: a standard difference < 10% indicates a negligible difference in the mean or prevalence of a covariate between two groups. Statistical analyses were performed using SPSS (the statistical package for social sciences) IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp R version 3.4.2, from the Comprehensive R Archive Network (R Core Team, 2020). The ‘MatchIt’ package version 3.0.2 was used to match the data based on the propensity score.
RESULTS
Between January 2012 and December 2018, 199 patients underwent radical hysterectomy for stage 1 cervical cancer (≤ 4cm). Of the surgery performed, 112 (56.3%) were performed by laparotomy, and 87 patients (43.7%) underwent MIRH. Of the patients who underwent MIRH surgery, 31 patients (35.6%) underwent robotic surgery, and the remaining 56 patients (64.4%) underwent surgery laparoscopically. Baseline and treatment characteristics of the study population prior to propensity matching have been described in Table 1. The median age of the cohort was 50 years with an interquartile range of 12 years. The majority of the patients had squamous cell carcinoma (74.40%) and had tumour size 2-4 cm (62.80%).
Demographic and disease characteristics (prior to propensity matching)
| Variable | Categories | Results, (n = 199) |
| Age, Median (IQR) | 50(12) | |
| Route of surgery | Open | 112(56.30%) |
| MIRH | 87(43.70%) | |
| Histology | SCC | 149(74.90%) |
| Adenocarcinoma/ Adenosquamous | 50(25.1%) | |
| Final Stromal Invasion | < 50% | 81(40.70%) |
| >/= 50% | 118(59.30%) | |
| LVSI | Absent | 136(68.30%) |
| Present | 63(31.70%) | |
| Parametria | Negative | 195(98.00%) |
| Positive | 4(2.00%) | |
| Vaginal_margins | Negative | 198(99.50%) |
| Positive | 1(0.50%) | |
| Radiation setting | Adjuvant radiation | 70(35.20%) |
| None | 126(63.30%) | |
| Defaulted | 3(1.50%) | |
| Type of radiation | None | 127(63.80%) |
| RT | 60(30.20%) | |
| CTRT | 7(3.50%) | |
| Not known | 5(2.50%) | |
| Tumour size | </= 2 CMS | 74(37.20%) |
| > 2 CMS TO </= 4 CMS | 125(62.80%) | |
| Stage_2018 | 1A1 | 11(5.50%) |
| 1A2 | 1(0.50%) | |
| 1B1 | 65(32.70%) | |
| 1B2 | 114(57.30%) | |
| 2A1 | 6(3.00%) | |
| 2B | 2(1.00%) | |
| Depth of stromal invasion | < 1/2 | 77(38.70%) |
| >/= 1/2 | 118(59.30%) | |
| Not applicable | 4(2.00%) | |
| Grade | 1 | 1(0.50%) |
| 2 | 88(44.40%) | |
| 3 | 101(51.00%) | |
| Risk_stratification | Low | 144(72.40%) |
| Intermediate | 50(25.10%) | |
| High | 5(2.50%) | |
Four disease characteristics which are known to independently affect survival, such as histology, tumour size, postoperative risk stratification, and adjuvant treatment received were used in propensity score matching and well balanced between open (n = 87) and MIRH group (n = 87) as shown in Table 2.
Clinical and pathological characteristics of the study population before and after propensity score matching.
| Variables | Cohort before propensity score matching | Cohort after propensity score matching | ||||||
| Histology n (%) | Total | Open | MIRH | P-value | Total | Open | MIRH | P-value |
| SCC | 149(74.90%) | 80(71.40%) | 69(79.30%) | 0.204 | 137(78.70%) | 68(78.20%) | 69(79.30%) | 0.853 |
| ADENOCARCINOMA | 50(25.10%) | 32(28.60%) | 18(20.70%) | 37(21.30%) | 19(21.80%) | 18(20.70%) | ||
| Tumour size n (%) | ||||||||
| </= 2 CMS | 74(37.20%) | 45(40.20%) | 29(33.30%) | 0.322 | 60(34.50%) | 31(35.60%) | 29(33.30%) | 0.281 |
| > 2 CMS TO </= 4 CMS | 125(62.80%) | 67(59.80%) | 58(66.70%) | 114(65.50%) | 56(64.40%) | 58(66.70%) | ||
| Risk_stratification | ||||||||
| Low | 144(72.40%) | 83(74.10%) | 61(70.10%) | 0.687 | 123(70.70%) | 62(71.30%) | 61(70.10%) | 0.598 |
| Intermediate | 50(25.10%) | 27(24.10%) | 23(26.40%) | 47(27.00%) | 24(27.60%) | 23(26.40%) | ||
| High | 5(2.50%) | 2(1.80%) | 3(3.40%) | 4(2.30%) | 1(1.10%) | 3(3.40%) | ||
| Radiation_setting | ||||||||
| Adjuvant radiation | 70(35.20%) | 43(38.40%) | 27(31.00%) | 0.281 | 57(32.80%) | 30(34.50%) | 27(31.00%) | 0.628 |
| None | 129(64.80%) | 69(61.60%) | 60(69.00%) | 117(67.20%) | 57(65.50%) | 60(69.00%) | ||
In the propensity-matched MIRH group, 63 patients have one of the two protective measures used. Thirty three patients had vaginal colpotomy without exposure of the cervical tumour to the peritoneal cavity and in 30 patients, no uterine manipulator was used. In 24 patients, no protective measures were used (patients had a uterine manipulator and also underwent abdominal colpotomy during MIRH procedure)
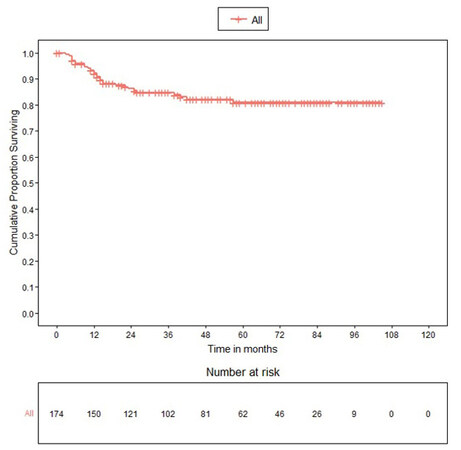
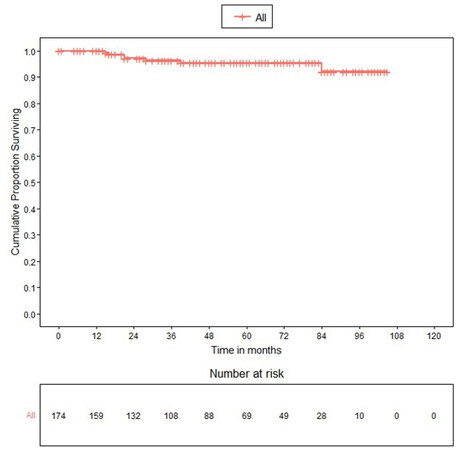
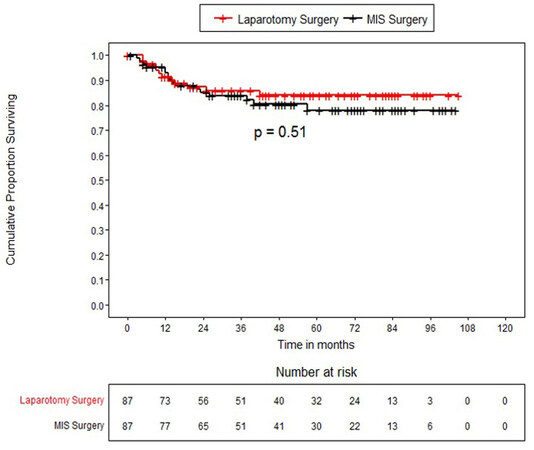
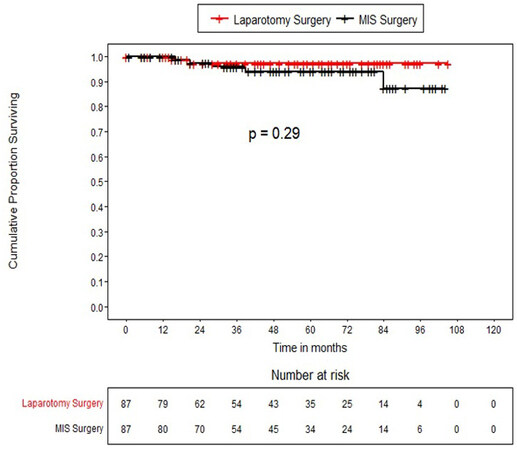
The median follow up of patients was 47 months (25th percentile 21 months to 73 months). Twenty-nine patients (16.7%) had recurrences, out of which 8.6% had local recurrence, 1% had only distant recurrence, and the rest had local with distant recurrence. Recurrences were similar in ORH and MIRH groups. For the entire propensity-matched cohort (n= 174), the DFS at 36 and 60 months was 84.8% (78.1%-89.6%) and 81% (73.4%-86.6%) respectively and the OS 96.5% (91.7%-98.5%) and 95.6% (90.3%-98%) respectively (Table 3). The DFS in patients who underwent open surgery at 36 and 60 months was 85.8 % (75.77%-91.92%) and 84.1% (73.47%-90.69%) respectively whereas, in patients who underwent MIRH surgery, DFS at 36 and 60 months was 83.9% (73.86%-90.33%) and 77.9% (65.94%-86.17%) respectively without any statistically significant difference between the study groups (log-rank P-value=0.510). The OS in patients who underwent open surgery at 36 and 60 months was 97.1 % (89.04%-99.28%) for both time periods, whereas OS in patients who underwent MIRH at 36 months and 60 months was 95.9% (87.77%-98.66%) and 94% (84.62%-97.74%) respectively without any statistically significant difference between the study groups (log-rank p-value=0. 293). Kaplan Meier survival curves for disease-free and overall survival for the overall matched cohort as well as for open and MIRH surgery are shown in Figure 1-4.
OS and DFS for the matched cohort (n = 174)
| Outcome | Survival (95% confidence interval) |
| Overall Survival | |
| 36 Months | 96.5% (91.7%-98.5%) |
| 60 Months | 95.6% (90.3%-98%) |
| Disease free survival | |
| 36 Months | 84.8% (78.1%-89.6%) |
| 60 Months | 81% (73.4%-86.6%) |
There was no statistical difference in DFS and OS when laparoscopic approach was compared with robotic approach (P = 0.376, 0.652 with 95%CI). However, the number of patients in laparoscopy (n = 56) was higher than robotic (n = 31).
In the MIRH cohort, 83.3% had no recurrence when no protective measures were used, and 81% had no recurrence when protective measures were used. There was no statistically significant difference in recurrence between the two groups.
DISCUSSION
Last couple of years has been contentious with regards to the route of surgery for radical hysterectomy in early cervical cancer. LACC trials and various retrospective studies published following LACC trials have shown mixed outcomes with MIRH[6,7,9-13,17-21]. However, the survival outcomes (OS, DFS) in earlier studies such as Landoni et al.[22], which made open radical surgery the standard of care for stage 1 disease in comparison to radiotherapy, was not as good as those presented in LACC trial. There could be various reasons for this observation, such as cancers arising in the then unscreened population altering the natural history of the disease, lack of advanced imaging modalities, stage migration and indications for adjuvant treatment along with improved open surgery techniques over a period of time[15,22,23].
In the recently published and extensively quoted trials such as LACC and SUCCOR[6,13], the mean age of the population was between 46 and 48 years. The median age in the present study is 50 years with an interquartile range of 12 years. In the present study, patients with tumour size < 2cm and between 2-4 cm were well distributed in both groups, with almost two-thirds (62.8%) of the patients having tumour sizes between 2 and 4 cm, which is higher as compared to 43% patients in LACC trial[6] and 58% patients in SUCCOR trial[13]. In the present study, there is a higher proportion of patients with grade 2 and 3 tumours and squamous cell carcinomas (74.9%) compared to recently published studies representing a real-world scenario of cancers arising in an unscreened population in developing countries[6,13].
Only one-third (32.8%) of the patients in the present study required adjuvant radiation, and cases were well distributed in both MIRH and open surgery groups without any statistical significance. This distribution is comparable to LACC study (approximately 28% in both groups) but less than the SUCCOR study (approximately 52% in both groups). In the present study, the numbers receiving concurrent chemoradiation (CTRT) (3.5%) is lesser compared to other published trials as lymph node-positive disease on frozen section did not undergo radical hysterectomy, and very few patients had microscopic metastatic emboli in parametrium and received CTRT.
Large size, adenocarcinoma and high-grade tumours are more aggressive and have higher metastatic potential and overall inferior oncological outcomes compared to squamous carcinomas and low-grade tumours[23]. Even though traditional risk factors found on histopathologies such as tumour size, type of histology and grade is incorporated in statistical analysis while calculating oncological outcomes, it is difficult to differentiate what factors specifically contribute to spreading or recurrence when the route of surgery is factored in, as sample size calculation is not based on this distribution.
In the present study, there is no statistically significant difference in DFS and OS at 36 months and 60 months in ORH vs. MIRH groups. There were 29 recurrences in the whole cohort. The patterns of recurrences were similar in ORH and MIRH groups (only local recurrence 7 and 8, distant + local recurrence 5 and 8 respectively).
With regards to inferior oncological outcomes and route of surgery, following LACC and SEER database publications in 2018 NEJM, a number of retrospective studies and metanalysis corroborated these findings[7-12]. However, recent large population based retrospective studies from Denmark, Sweden, and the Netherlands[18-20] and a single-institutional retrospective study from India[21] failed to show differences in DFS and OS between ORH and MIRH.
The probable reasons cited for poor oncological outcomes with MIRH in LACC and other retrospective studies following LACC trial were the use of a uterine manipulator, prolonged steep Trendelenburg position and intracorporeal vaginal colpotomy in the setting of high-pressure pneumoperitoneum contributing to the dissemination of tumour cells in the peritoneal cavity especially in tumours > 2cms. In SUCCOR study, although the overall risk of recurrence and death for patients who underwent MIRH was twice as high as ORH, the patients with tumour size more than 2 cm who underwent MIRH with protective vaginal closure methods and without the use of uterine manipulator had similar rates of relapse compared to those who underwent ORH. Extrapolating patients from SUCCOR study database, Chacon et al.[24] also showed that patients who had prior cervical conisation had a 72 % reduction in risk of relapse and 90% decrease in risk of death, and this effect was more evident in those with tumours 2-4 cm in size in MIRH group. Uppal et al.[10]. although showed significantly inferior DFS in MIRH for tumours ≤ 2 cm, they also showed that conisation before surgery was associated with lower recurrence risk. Many techniques have been tried to prevent tumour spillage into the peritoneal cavity by various authors like Köhler et al.’s[25] ‘transvaginal closure technique of vaginal cuff. Kanao et al.’s[26] ‘no-look no-touch technique’, Li Jinjin’s tie combined with a cuppy uterine manipulator method[27].
All the current evidence highlights the importance of tumour containment and refining existing techniques to prevent tumour spillage and dissemination into the peritoneal cavity in the setting of high-pressure pneumoperitoneum during MIRH surgery, although it needs to be proven in future prospective studies. This observation might also throw light on the fact that cancers contained inside the walls of the organs such as the endometrium and colon have shown non-inferior oncological outcomes with minimally invasive surgeries compared to the cervix where the friable growth is exposed to the peritoneal cavity[28-30].
In the present study, the type of recurrences was similar in ORH vs. MIRH. Two-thirds (72.4%) had one form of the protective measures used during MIRH (vaginal colpotomy/no uterine manipulator). This could also have contributed to non-inferior survival in MIRH group, but could not be proved in this study due to small subgroups with no protective measure.
The present study is from a large developing country comparing ORH and MIRH (laparoscopy and Robotic), where the cervical cancer burden is high, and the majority of the population is unscreened and unvaccinated for cervical cancer prevention. The tertiary cancer centre in which the study is conducted caters to all socioeconomic strata of society, representing the true economic demographics and disease epidemiology amongst them. Confounding factors in the aetiology of cervical cancer and the natural history of human papillomavirus (HPV) carcinogenesis might be different between unscreened and screened populations and should be taken into consideration given the fact that the global cervical cancer burden is largely from developing countries with economic disparity.
One of the criticisms in the LACC trial published by the NEJM editorial was that the vast majority of patients with recurrences concentrated in 14 out of the 33 recruiting centres[31], questioning the standardisation and surgical load needed to maintain technical expertise. The present study is a single institution study from one of the largest tertiary cancers centres established over 75 years, with excellent surgical load and surgical expertise and a pioneering national subspecialty training program ensuring the quality of open and MIRH surgery. The disease characteristics which would potentially affect survival, such as histology, tumour size, postoperative risk stratification, and adjuvant treatment received, were well balanced between ORH and MIRH group on propensity score matching. Most data on equivalent outcomes with regards to surgical technique is supportive of less than 2 cm tumours[6,13] and not of 2-4 cm tumours, making present study unique as two-thirds of the cohort had 2-4 cm size tumour. Drawbacks of the current study include inherent shortcomings of an observational retrospective study and not addressing the quality of life in patients undergoing radical hysterectomy.
At present, there are two prospective randomised trials exploring the role of MIRH in patients with cervical cancer. The first is the RACC trial (robotic-assisted approach to cervical cancer) by Falconer et al.[32], a Swedish prospective multicentric trial is comparing robotic vs. open surgery for the treatment of early-stage cervical cancer. The second trial is a multicentre randomised controlled trial designed in China by Chao et al.[33].
In conclusion, proper patient selection of lymph-node negative ≤ 4cms cervical tumours along with appropriate training and expertise will improve outcomes for surgery for cervical cancer.
Using safe practices to prevent tumour spill is a good clinical practice for all cancers, irrespective of its effect on oncological outcomes. Refining existing techniques to prevent tumour spill and potential peritoneal recurrence needs to be proved in prospective studies. Audits on oncological outcomes in individual centres should dictate the route of surgery with adequate counselling of patients before offering ORS or MIRH till robust data on technique and technology is available.
Future studies should be planned to compare the route of surgery and also screening facilities in a country which will exclude the bias of natural history of HPV carcinogenesis/precancer in treated and untreated populations.
DECLARATIONS
Authors’ contributionsMade substantial contributions to conception and design of the study, performed data acquisition, analysis and interpretation, have drafted and substantively revised the work: Shylasree TS
Performed data acquisition, analysis and interpretation, have drafted and substantively revised the work: Gupta S
Made substantial contributions to the design of the study, data analysis and interpretation: Patil A
Have drafted and substantively revised the work: Singh P, Maheshwari A, Menon S, Chopra S, Gurram L, Popat P, Mahantshetty U, Kerkar R
Availability of data and materialsThe data that support the findings of this study have been submitted to the institutional review board of Tata Memorial Hospital, Mumbai, India and are available from the corresponding author, upon reasonable request within confines of patient confidentiality, ethics and law.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateThe study was accepted by the institutional review board (OIEC/3687/2021/00004) who deemed separate study-specific consent is not needed.
Consent for publicationNot applicable
Copyright© The Author(s) 2021.
REFERENCES
1. Gallotta V, Conte C, Federico A, et al. Robotic versus laparoscopic radical hysterectomy in early cervical cancer: a case matched control study. Eur J Surg Oncol 2018;44:754-9.
2. Zhao Y, Hang B, Xiong GW, Zhang XW. Laparoscopic radical hysterectomy in early stage cervical cancer: a systematic review and meta-analysis. J Laparoendosc Adv Surg Tech A 2017;27:1132-44.
3. Park DA, Yun JE, Kim SW, Lee SH. Surgical and clinical safety and effectiveness of robot-assisted laparoscopic hysterectomy compared to conventional laparoscopy and laparotomy for cervical cancer: a systematic review and meta-analysis. Eur J Surg Oncol 2017;43:994-1002.
4. Hong JH, Choi JS, Lee JH, et al. Can laparoscopic radical hysterectomy be a standard surgical modality in stage IA2-IIA cervical cancer? Gynecol Oncol 2012;127:102-6.
5. Ramirez PT, Soliman PT, Schmeler KM, dos Reis R, Frumovitz M. Laparoscopic and robotic techniques for radical hysterectomy in patients with early-stage cervical cancer. Gynecol Oncol 2008;110:S21-4.
6. Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med 2018;379:1895-904.
7. Doo DW, Kirkland CT, Griswold LH, et al. Comparative outcomes between robotic and abdominal radical hysterectomy for IB1 cervical cancer: Results from a single high volume institution. Gynecol Oncol 2019;153:242-7.
8. Kim SI, Lee M, Lee S, et al. Impact of laparoscopic radical hysterectomy on survival outcome in patients with FIGO stage IB cervical cancer: a matching study of two institutional hospitals in Korea. Gynecol Oncol 2019;155:75-82.
9. Cusimano MC, Baxter NN, Gien LT, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol 2019;221:619.e1-619.e24.
10. Uppal S, Gehrig PA, Peng K, et al. Recurrence rates in patients with cervical cancer treated with abdominal versus minimally invasive radical hysterectomy: a multi-institutional retrospective review study. J Clin Oncol 2020;38:1030-40.
11. Chen X, Zhao N, Ye P, et al. Comparison of laparoscopic and open radical hysterectomy in cervical cancer patients with tumor size ≤ 2 cm. Int J Gynecol Cancer 2020;30:564-71.
12. Nitecki R, Ramirez PT, Frumovitz M, et al. Survival after minimally invasive vs open radical hysterectomy for early-stage cervical cancer: a systematic review and meta-analysis. JAMA Oncol 2020;6:1019-27.
13. Chiva L, Zanagnolo V, Querleu D, et al. SUCCOR study Group. SUCCOR study: an international European cohort observational study comparing minimally invasive surgery versus open abdominal radical hysterectomy in patients with stage IB1 cervical cancer. Int J Gynecol Cancer 2020;30:1269-77.
14. Sreedevi A, Javed R, Dinesh A. Epidemiology of cervical cancer with special focus on India. Int J Womens Health 2015;7:405-14.
15. Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a gynecologic oncology group study. Gynecol Oncol 1999;73:177-83.
16. Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 2000;18:1606-13.
17. Kim SI, Cho JH, Seol A, et al. Comparison of survival outcomes between minimally invasive surgery and conventional open surgery for radical hysterectomy as primary treatment in patients with stage IB1-IIA2 cervical cancer. Gynecol Oncol 2019;153:3-12.
18. Wenzel HHB, Smolders RGV, Beltman JJ, et al. Survival of patients with early-stage cervical cancer after abdominal or laparoscopic radical hysterectomy: a nationwide cohort study and literature review. Eur J Cancer 2020;133:14-21.
19. Jensen PT, Schnack TH, Frøding LP, et al. Survival after a nationwide adoption of robotic minimally invasive surgery for early-stage cervical cancer - a population-based study. Eur J Cancer 2020;128:47-56.
20. Alfonzo E, Wallin E, Ekdahl L, et al. No survival difference between robotic and open radical hysterectomy for women with early-stage cervical cancer: results from a nationwide population-based cohort study. Eur J Cancer 2019;116:169-77.
21. Sekhon R, Naithani A, Makkar P, et al. Robotic radical hysterectomy versus open radical hysterectomy for cervical cancer: a single-centre experience from India. J Robot Surg 2021; doi: 10.1007/s11701-021-01320-6.
22. Landoni F, Maneo A, Colombo A, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997;350:535-40.
23. Landoni F, Colombo A, Milani R, Placa F, Zanagnolo V, Mangioni C. Randomized study between radical surgery and radiotherapy for the treatment of stage IB–IIA cervical cancer: 20-year update. J Gynecol Oncol 2017;28:1-10.
24. Chacon E, Manzour N, Zanagnolo V, et al. SUCCOR study group. , SUCCOR study Group. SUCCOR cone study: conization before radical hysterectomy. Int J Gynecol Cancer 2022;32:117-24.
25. Kohler C, Hertel H, Herrmann J, et al. Laparoscopic radical hysterectomy with transvaginal closure of vaginal cuff - a multicenter analysis. Int J Gynecol Cancer 2019;29:845-50.
26. Kanao H, Matsuo K, Aoki Y, et al. Feasibility and outcome of total laparoscopic radical hysterectomy with no-look no-touch technique for FIGO IB1 cervical cancer. J Gynecol Oncol 2019;30 :1-12. [PMID: 30887768 DOI: 10.3802/jgo.2019.30. e71].
27. Li J, Ouyang X, Gong X, et al. Survival outcomes of minimally invasive surgery for early-staged cervical cancer: a retrospective study from a single surgeon in a single center. Asian J Surg 2022;45:320-5.
28. Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: gynecologic oncology group LAP2 study. J Clin Oncol 2012;30:695-700.
29. Bonjer HJ, Deijen CL, Abis GA, et al. COLOR II Study Group. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015;372:1324-32.
30. Janda M, Gebski V, Davies LC, et al. Effect of total laparoscopic hysterectomy vs total abdominal hysterectomy on disease-free survival among women with stage I endometrial cancer: a randomized clinical trial. JAMA 2017;317:1224-33.
31. Kong TW, Chang SJ, Paek J, Park H, Kang SW, Ryu HS. Learning curve analysis of laparoscopic radical hysterectomy for gynecologic oncologists without open counterpart experience. Obstet Gynecol Sci 2015;58:377-84.
32. Falconer H, Palsdottir K, Stalberg K, et al. Robot-assisted approach to cervical cancer (RACC): an international multi-center, open-label randomized controlled trial. Int J Gynecol Cancer 2019;29:1072-6.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Shylasree TS, Gupta S, Patil A, Singh P, Maheshwari A, Menon S, Chopra S, Gurram L, Popat P, Mahantshetty U, Kerkar R. Surgical route and pathological risk factors in early cervical cancer - Node Zero (SURPEC-N0). J Cancer Metastasis Treat 2022;8:14. http://dx.doi.org/10.20517/2394-4722.2022.10
AMA Style
Shylasree TS, Gupta S, Patil A, Singh P, Maheshwari A, Menon S, Chopra S, Gurram L, Popat P, Mahantshetty U, Kerkar R. Surgical route and pathological risk factors in early cervical cancer - Node Zero (SURPEC-N0). Journal of Cancer Metastasis and Treatment. 2022; 8: 14. http://dx.doi.org/10.20517/2394-4722.2022.10
Chicago/Turabian Style
Shylasree, T S, Stuti Gupta, Akshay Patil, Pooja Singh, Amita Maheshwari, Santosh Menon, Supriya Chopra, Lavanya Gurram, Palak Popat, Umesh Mahantshetty, Rajendra Kerkar. 2022. "Surgical route and pathological risk factors in early cervical cancer - Node Zero (SURPEC-N0)" Journal of Cancer Metastasis and Treatment. 8: 14. http://dx.doi.org/10.20517/2394-4722.2022.10
ACS Style
Shylasree, TS.; Gupta S.; Patil A.; Singh P.; Maheshwari A.; Menon S.; Chopra S.; Gurram L.; Popat P.; Mahantshetty U.; Kerkar R. Surgical route and pathological risk factors in early cervical cancer - Node Zero (SURPEC-N0). J. Cancer. Metastasis. Treat. 2022, 8, 14. http://dx.doi.org/10.20517/2394-4722.2022.10
About This Article
Copyright
Data & Comments
Data

 Cite This Article 11 clicks
Cite This Article 11 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.