The role of VISTA in the tumor microenvironment
Abstract
V-domain Ig Suppressor of T cell Activation (VISTA) is a negative immune checkpoint that is expressed on multiple immune cell subsets and has been characterized in T cells, macrophages, and myeloid-derived suppressor cells. As the only immune checkpoint expressed on naïve T cells, VISTA contributes to the maintenance of T cell quiescence and tolerance. VISTA also regulates multiple myeloid cell activities such as chemotaxis, differentiation, and migration. In the context of cancer, antagonistic monoclonal antibody targeting of VISTA has been shown to aid anti-tumor immunity. Furthermore, combination therapies that include other immune checkpoints such as PD-1 or CTLA-4 with VISTA blockade may enhance therapeutic efficacy in a variety of cancers. Combination therapy may help overcome adaptive resistance to individual checkpoint therapies, thereby improving patient outcomes and survival. Here, we summarize the role of VISTA in myeloid cells and T cells within the tumor microenvironment. We discuss the proposed counter-receptors for VISTA, VISTA antibodies currently in development, and the potential for combination therapies with checkpoint inhibitors such as PD-1 and CTLA-4.
Keywords
INTRODUCTION
While cancer mortality rates have decreased over the past several decades due to the classic cancer treatment approaches (chemotherapy, radiation, and surgery), incidence rates continue to climb and are predicted to increase by 49% in the United States between 2015 and 2050[1]. New therapeutic strategies are necessary to counteract disease burden, particularly in patients whose cancers resist treatment. Today, the complexity of the tumor microenvironment (TME) is appreciated, and it is well established that the TME promotes tumor growth, immune evasion, and resistance to therapies[2]. The TME consists of tumor cells (TCs), stromal cells (SCs), and immune cells (ICs). These cellular compartments of the TME vary in patients depending on factors such as cancer type and stage, expression of immune checkpoints, and treatment resistance. Characterizing the cellular contributions of the TME to tumor progression is critical in optimizing treatment strategies to overcome resistance and increase patient survival.
Harnessing the immune system for cancer therapies has gained popularity in recent decades. Cancer immunotherapies include antibodies, cellular therapies, cytokine-based therapies, small molecules, vaccines, and immune checkpoint inhibitors (ICIs). Targeting the immune system with ICIs yielded stark improvements in survival rates in preclinical studies, but clinical responses are more modest, in part due to the highly immunosuppressive TME[2-4]. This review focuses on the role of the immune checkpoint V-domain Ig Suppressor of T cell Activation (VISTA) in the TME.
Immune checkpoints can regulate the immune system on multiple levels and are particularly notable in their regulation of T cell activation to prevent activation-induced damage to tissue. In the highly immunosuppressive TME, dysfunctional T cells express immune checkpoints[5,6]. Therefore, current therapies target these immune checkpoints to relieve T cell suppression[7]. Major pathways targeted by ICIs in the clinic include those that target programmed cell death protein-1 (PD-1) and programmed death-ligand 1 (PD-L1), engaged during exhaustion; and cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), induced after activation.
While the approval of ipilimumab in 2011, an anti-CTLA-4 monoclonal antibody (mAb) revolutionized cancer treatment, ICI treatments have shown efficacy in only a subset of patients. A phase III clinical trial comparing anti-PD-1 (nivolumab) and anti-CTLA-4 (ipilimumab) showed that these drugs are efficacious in 44% and 19% of patients, respectively, and 58% when used in combination[8]. More than half of patients do not respond to immunotherapy at all (primary resistance) or stop responding to ICIs (acquired resistance). In both cases, their immune systems recognize the cancer but cannot attack it due to the cancer’s mechanisms of immune evasion (adaptive resistance)[2]. Adaptive resistance can arise after treatment with anti-PD-1 and/or anti-CTLA-4 and is associated with an upregulation in the immune checkpoint V-domain Ig Suppressor of T cell Activation (VISTA) in the TME[9-11]. This indicates that VISTA may be involved in the development of compensatory mechanisms of adaptive resistance, thus supporting the need for targeting VISTA to improve therapeutic outcomes.
VISTA has been identified as a key player in regulating T cell activity, as well as in maintaining myeloid suppressiveness in the TME[12-14]. VISTA was first described as a member of the B7 class of immunoglobulin proteins that includes the ligands CD80, CD86 and PD-L1[15]. In the B7 family, VISTA is most closely related to PD-L1, with the two immune checkpoints sharing 24% sequence identity[15]. B7 proteins typically have both an Ig-V and an Ig-C domain, but VISTA has a single extracellular Ig-V domain, characteristic of the CD28 family receptor proteins. VISTA’s extracellular domain is unusual compared to other Ig domains due to its two extra disulfide bonds; a unique charged and extended loop; an additional beta strand; and an extra helix in the positively charged face of VISTA’s extracellular domain[16,17].
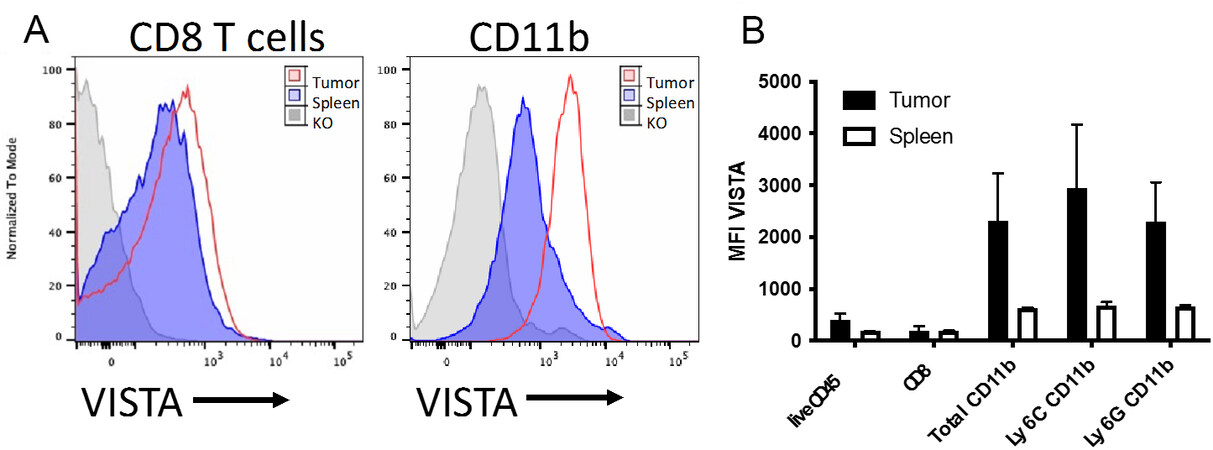
VISTA is widely expressed in the TME, including on TCs, SCs, and ICs. VISTA expression on TCs varies greatly depending upon the cancer type[18]. VISTA is broadly expressed in multiple IC lineages [Figure 1], including T cells, macrophages, monocytes, neutrophils, and myeloid-derived suppressor cells (MDSCs)[15,19,20]. While B cells do not express VISTA[19], we observed expression on plasma cells[21]. Only a small subset of CD56hi natural killer (NK) express VISTA[19]. Unlike other immune checkpoints whose expression is selectively induced on particular IC subsets, most ICs constitutively express VISTA. This makes VISTA a desirable therapeutic target for therapeutic intervention because of its broad expression in the TME and its ability to widely modulate the immune system.
Figure 1. VISTA expression in the periphery versus the tumor microenvironment. BALB/c mice were injected intradermally with 100k CT26 cells and were grown for two weeks. Tumors and spleens were dissociated and processed for flow cytometry. Cells from VISTA-deficient mice were used for staining controls. (A) Overlays showing VISTA expression on CD8+ T cells in the spleen or tumor. (B) VISTA expression as Mean Fluorescence intensity in spleen versus tumor within the indicated cell subsets. Data are represented as mean ± SD. VISTA: V-domain Ig Suppressor of T cell Activation.
Antibodies to VISTA can improve or dampen the immune response. Agonistic anti-VISTA enhances T cell tolerance, elicits a non-inflammatory program in macrophages and inhibits myeloid chemotaxis[12,22]. Consequently, VISTA agonism has been shown to ameliorate autoimmune disease in several preclinical murine models. Using models of VISTA agonism or deficiency, multiple studies have demonstrated the therapeutic relevance of VISTA targeting in experimental autoimmune encephalomyelitis[23], systemic lupus erythematosus[23-25], psoriasis[26] , and graft versus host disease[13,27]. In contrast, anti-VISTA antagonists dampen the establishment of T cell tolerance, exacerbate inflammation, and enhance anti-tumor immune responses. Preclinical murine cancer studies demonstrate slowed tumor growth when anti-VISTA is used as a monotherapy in multiple cancer models[9]. Enhanced tumor rejection has been observed when anti-VISTA is used in conjunction with anti-PD-1 or anti-PD-L1[28]. Combination therapies of multiple immune checkpoints holds promise for better clinical outcomes for patients.
VISTA EXPRESSION AND VALUE AS A BIOMARKER WITHIN THE TME
Different tumor types can have differing levels of VISTA expression. Cancer types with high VISTA expression include hepatocellular carcinoma[29,30], gastric cancers[31,32], colorectal cancer, prostate cancer[11], non-small cell lung cancer (NSCLC)[33], ovarian cancer[34], endometrial cancer[35], and gestational trophoblastic neoplasia[36]. VISTA expression in the TME is associated with poor prognosis in melanoma[37,38], oral squamous cell carcinoma (in cases of low CD8 expression)[39], pancreatic cancer[40], and gliomas[41]. While VISTA expression in the tumor may suppress immune cells and support tumor growth, its presence on the tumor could also indicate an active anti-tumor immune response and be a positive prognostic factor. In support of this, a meta-analysis of solid tumors found that high VISTA expression in tumors was associated with high levels of T cell infiltration and improved overall survival[42]. High VISTA expression in the TME can be coupled with high levels of T cell infiltration and increased overall survival, as seen in esophageal adenocarcinoma[43], hepatocellular carcinoma[30] and triple-negative breast cancer[44]. Other cancers in which VISTA expression in the TME is associated with a positive prognosis include PDAC[40], non-small cell lung cancer[33,45] and high-grade serous ovarian cancer[46].
While VISTA expression is predominantly immune associated (as discussed later in the review), recent studies have highlighted that in some cancers, VISTA may be expressed outside of the hematopoietic compartment on either TCs or SCs (including adipocytes, endothelial cells, fibroblasts, and stellate cells). Expression of VISTA on TCs is positively associated with patient outcome in ovarian cancer[34], hepatocellular carcinoma[30], and NSCLC[33], while in colorectal cancer[47] and gastric cancer[31], no link with objective survival was observed.
On the other hand, VISTA expression on vascular endothelial cells is associated with lymph node metastasis in cervical[48] and ovarian cancer[34]. Beyond their association with poor prognosis, SCs have been associated with influencing ICs in the TME[49]. Melanoma-associated fibroblasts had increased VISTA expression relative to fibroblasts isolated from noncancerous intact edges of the tumor, and they can interfere with intracellular CTL signaling[50]. Additionally, distribution of immune cell infiltration in tumor versus stromal regions of the TME can vary depending on the tumor, potentially impacting therapeutic response[51]. Much like VISTA expression on ICs, the role of VISTA in non-hematopoietic cells such as TCs and SCs likely varies between cancer types and patients, so further studies are required to determine which markers might be positive indicators for VISTA antagonism.
For cancers in which VISTA expression is a positive prognostic factor, VISTA blockade may still have therapeutic benefits. Much like PD-L1 expression[52], negative suppressive impact of VISTA within the TME could be masked by the positive association with immune infiltration[42]. Even in tumors where VISTA expression is low or not directly associated with prognosis or survival—such as oral squamous sarcoma[53], ovarian cancer[35], prostate cancer, and renal cell carcinoma[54]—VISTA blockade may propel the TME from a “cold” tumor with poor IC infiltration, to become a “hot” tumor with high IC infiltration.
THE ROLE OF VISTA ON MYELOID CELLS
Myeloid cells highly express VISTA, differentiating VISTA from other immune checkpoints. Notably, VISTA expression on myeloid cells in the TME is much higher than that of myeloid cells in the periphery [Figure 1]. VISTA is highly expressed on monocytes and regulates monocyte migration and activation. One pathway that is responsible for this is the CCL2/CCR2 axis, which recruits inflammatory monocytes to the TME, thus playing a critical role in cancer development and metastasis[55]. In splenic monocytes and bone-marrow derived-monocytes from VISTA-deficient mice, surface expression of CCR2 is significantly reduced, thus impairing monocyte migration[12]. VISTA may therefore regulate monocyte recruitment to the TME via the CCL2/CCR2 axis. VISTA could also be regulating monocytes through other inflammatory pathways, such as the NFκB1 pathway that is significantly downregulated in human monocytes after treatment with a VISTA agonist[22].
A potential mechanism for anti-PD-1 or anti-CTLA-4 treatment failure is MDSC escape mechanisms. Such escape mechanisms include MDSC accumulation, activation, trafficking, and T cell inhibition[56,57]. In the B16 OVA model, MDSC levels decrease in mice treated with an antagonistic VISTA mAb known as 13F3
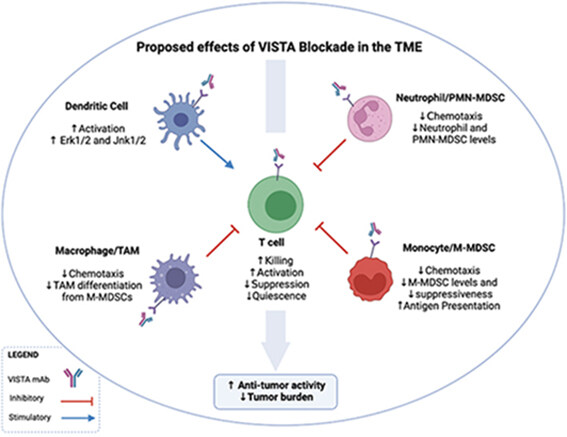
Figure 2. The proposed effects of VISTA blockade in the TME. The administration of an anti-VISTA antagonist results in the recruitment of T cells to the TME, as well as a reduction in chemotaxis of MDSCs and macrophages into the TME. VISTA antagonism also results in decreased differentiation of MDSCs into TAMS is also decreased; reduced production of suppressive cytokines by MDSCs and macrophages, resulting in decreased T cell inhibition, and increased production of IFNγ and IL-2. DCs become more activated and upregulate Erk1/2 and Jnk1/2, potentially regulating the IL-23/IL-17 inflammatory axis. Neutrophil and PMN-MDSC levels are decreased in the TME by the reduction of chemotaxis after VISTA blockade. Altogether, these effects of VISTA antagonism result in reduced tumor burden. VISTA: V-domain Ig Suppressor of T cell Activation; TME: tumor microenvironment; MDSCs: myeloid-derived suppressor cells; TAMS: tumor associated macrophages.
Tumor associated macrophages (TAMs) infiltrate the TME and contribute to tumor cell proliferation, angiogenesis, and metastasis[59]. VISTA deficiency impairs macrophage chemotaxis, migration, and cytokine production [Figure 2][12,60]. Moreover, VISTA KO macrophages produce high amounts of inflammatory cytokines such as CCL2, CCL3, CCL4, and CCL5 at steady state and after LPS stimulation[12], highlighting the role of VISTA in macrophage suppressiveness and potential regulatory role of VISTA antagonism of TAMs in the TME. Interestingly, VISTA agonist induce macrophage tolerance and promote anti-inflammatory pathways[22]. VISTA deficiency also decreases TAM levels in the TME of CT26 tumor-bearing mice, consistent with the data demonstrating that M-MDSCs can differentiate into TAMs [Figure 2] and potentially inflammatory DCs[12,61].
VISTA expression is associated with MDSCs in cutaneous melanoma[38], oral squamous cell carcinoma[39], and acute myeloid leukemia[62], suggesting that these cancers are strong candidates for VISTA blockade. For example, expression of VISTA on MDSCs and expression of PD-1 on CD8+ T cells, CD4+ T cells, and Treg are positively correlated in acute myeloid leukemia (AML) patients[62]. Blocking this interaction could have a therapeutic impact by alleviating MDSC suppressiveness and enhancing T cell responses. Together, these data support that VISTA expression on MDSCs modulates the TME, and that VISTA blockade has therapeutic benefits, particularly in the context of adaptive resistance.
Another myeloid cell subset that expresses VISTA is monocytic-DCs (mDCs), which are associated with cancer inflammation and can arise as a result of M-MDSC differentiation. VISTA antagonism in the B16F10 melanoma model increases the activation state of DCs, resulting in increased expression of CD80 and MHCII, as well as increased production of cytokines such as IL-12p40 and TNFα[9,63]. VISTA may also regulate DCs by suppressing Erk1/2 activation and by inhibiting IL-23 production[26]. Therefore, VISTA antagonism may reverse immune suppression and enhance anti-tumor responses.
THE ROLE OF VISTA ON GRANULOCYTES
Beyond the previously discussed subsets, VISTA is also expressed on granulocytes, including eosinophils[64], neutrophils[19], and PMN-MDSCs[63]. Despite being associated with allergic diseases, some tumors display eosinophilia[65]. Eosinophils express VISTA constitutively at low levels but have been shown to nonetheless affect immune responses[64]. In an OVA-induced asthma model, VISTA-deficient mice had significantly higher eosinophil levels in the lung compared to WT mice[66]. Another group showed that the use of an antagonistic VISTA mAb during antigen challenge induced an eosinophil mediated inflammatory Th2 allergy response. Together, these studies suggest that VISTA blockade may help modulate eosinophils in the TME to improve patient outcomes.
VISTA also plays a regulatory role in neutrophils. IHC analysis of primary cutaneous melanoma samples demonstrated that VISTA expression correlates with neutrophil infiltration of the TME and that neutrophils were typically associated with tumor ulceration and necrosis[37]. Transcriptional analysis of
Indeed, VISTA antagonism significantly reduces PMN-MDSC levels in the TME and spleen in a murine B16F10 melanoma model[9]. However, treatment did not decrease suppressive activities of PMN-MDSCs as it did with M-MDSCs[9]. Though PMN-MDSCs and M-MDSCs are different in origin and function, both subsets are suppressive in the TME and tend to be poor prognostic factors in cancer[68,69]. These data indicate that the PMN-MDSC subset might not directly respond to VISTA blockade alone and can be taken into consideration when designing a combination therapy approach.
VISTA: A DIRECT AND INDIRECT REGULATOR OF T CELLS IN THE TME
VISTA acts as a negative checkpoint regulator of naïve T cell quiescence and peripheral tolerance[14]. Both naïve and memory CD4+ and CD8+ T cells express VISTA, and multiple studies show that VISTA modulates T cell activity[20,23]. Aged VISTA-deficient mice display enhanced T cell activation and cytokine production[23]. VISTA-/- CD4s cultured in vitro with anti-CD3 also exhibit high proliferation and production of cytokines such as IFNγ, IL-17A and TNFα[20]. Furthermore, plate-bound VISTA Ig inhibits CD4+ and CD8+ T cell proliferation and cytokine production, as well as reduces IL-2 production by CD4+ memory T cells and IFNγ production by CD8+ T cells[15].
Naïve CD4+ T cells exhibit VISTA mediated control of quiescence and peripheral tolerance[14]. Single-cell sequencing of naïve CD4+ T cells shows unexpected heterogeneity in the naïve T cell compartment, with VISTA-/- mice showing a loss in a cluster marked by genes involved in T cell quiescence[14]. CD4+ T cells are also less tolerized against self-peptides[14]. These data indicate that VISTA alters T cell tolerance, so targeting VISTA holds promise in modulating T cell tolerance to tumors. The change in tolerance may allow for a broader repertoire of T cells to be recruited into the TME, resulting in a more robust and broader anti-tumor immune response. We speculate that VISTA signaling therefore may work in conjunction with TCR signaling by potentially reducing the threshold of TCR signaling and allowing for lower affinity antigens with that to induce immune responses. Naïve T cell regulation by VISTA may therefore be utilized to improve cancer therapies.
In mice bearing B16OVA melanoma tumors, VISTA blockade using the VISTA antagonist monoclonal antibody 13F3 results in enhanced T cell activation and increased anti-tumor responses via recruitment of immune cells to the TME[9]. 13F3 has also been shown to have anti-tumor effects on other tumors, including the bladder tumor model MB49 and B16BL6 melanoma model[9]. In the GL261 glioma tumor model, VISTA-induced reduction of disease burden in a CD4+ T cell-dependent manner, as shown by CD4 depletion experiments[20]. Remarkably, the combination of anti-VISTA and a single dose of a peptide vaccine resulted in an even greater reduction of tumor burden[9]. The authors suggest that high VISTA expression on myeloid cells within the TME results in effector T cell suppression, as well as recruitment of immunosuppressive Tregs and M-MDSC to the TME [Figure 2][9]. Tregs, a major contributor to immune suppression during cancer, can also be modulated by VISTA targeting[9]. Upon adoptive transfer of OTIIs into B16OVA-bearing mice, CD4+ conversion to Tregs was reduced in mice that received 13F3[9]. Targeting VISTA in tumors therefore may have a direct effect on T cell activity and recruitment, as well as display an indirect effect on T cells via MDSCs or induced Treg differentiation[70].
PD-1 and CTLA-4 are important checkpoint regulators of the immune system currently being targeted for cancer therapies in the clinic. These pathways work in a non-redundant manner on T cells compared to VISTA[23,28]. Studies comparing the changes in T cell effector pathways of tumor-specific CD8+ T cells found that anti-CTLA-4 promotes cell cycle and effector memory responses, while anti-PD-1 enhances metabolism and effector function[67]. Further, while anti-CTLA-4 induces the expansion of ICOS+ Th1-like CD4+ T cells, anti-PD-1 drives re-expansion of exhausted CD8+ T cell subsets[71]. The combination of anti-PD-1 and anti-CTLA-4 promotes progenitor exhausted (Tex-prog) subsets, which are polyfunctional and retain proliferative capacity[72,73]. While these checkpoints target T cell priming or exhaustion, anti-VISTA has the unique ability to target naïve T cells, as well as potentially modulate T cell activity indirectly via other immune cells present in the TME[14]. VISTA can therefore be used in combination with anti-PD-1 or anti-CTLA-4 to enhance T cell activity and therapeutic outcomes in a non-redundant manner[28] [Table 1].
Predicted outcomes of VISTA blockade with PD-1 or CTLA-4 blockade
| Co-blockade | Immune checkpoint expression in the TME and relevant data | Proposed impact on the TME |
| VISTA + | VISTA on MDSCs associates with PD-1 on T cells in AML[64] VISTA expression ↑ on intratumoral lymphocytes in metastatic melanoma after anti-PD-1 monotherapy or anti-PD-1 and anti-CTLA-4 combination therapy[10] VISTA expression associates with PD-1 and PD-L1 in NSCLC[46], craniopharyngioma[75], and ENKTCL[76] | Co-blockade of VISTA and PD-1 reduces the likelihood of developing resistance by blocking VISTA-related compensatory immune escape pathways in myeloid and T cells |
| VISTA + CTLA-4 | High VISTA expression on TAMs and CD68+ macrophages in pancreatic cancer and PDAC, respectively[40,55] In OSC, VISTA is overexpressed in tumor-infiltrating ICs and associated with PD-L1 and CTLA-4 expression[39] VISTA expression ↑ in tumor tissue, blood monocytes, and tumor-associated CD4+ and CD8+ T cells in PC after anti-CTLA-4 treatment[11] | Co-blockade of VISTA and CTLA-4 may reduce adaptive resistance to anti-CTLA-4, and have complementary impacts on T cell activation because VISTA regulates T cells prior to activation and CTLA-4 regulates T cells after activation |
VISTA AND ITS COUNTER-RECEPTORS: THE TME
Multiple ligands are proposed to bind to VISTA, including PSGL-1[74], VSIG-3[75], syndecan-2[76], and even VISTA itself[77], suggesting that - like other B7 proteins - VISTA may have multiple binding partners.
V-set and immunoglobulin domain-containing protein 3 and V-set and immunoglobulin domain-containing protein 8 (VSIG-8) are members of the immunoglobulin superfamily and proposed as binding partners to VISTA[75,82]. However, subsequent studies have not found significant binding of VSIG-8 to VISTA[75]. While these proteins may bind VISTA under certain conditions, the biological relevance of these interactions as counter-receptors for VISTA is not yet clear, as VSIG-3 and VISG-8 are minimally expressed in secondary lymphoid organs where VISTA interactions are expected to occur[83]. In multiple tumor models (B16F10, MC38, and 4T1), SG7, an antibody that prevents both PSGL-1 and VSIG-3 from binding to VISTA, slowed tumor growth[84]. Unlike other VISTA antibodies that have demonstrated an Fc crosslinking requirement, this clone is Fc-silent[84]. Combination therapy of SG7 and anti-PD-1 resulted in a synergistic effect in the MC38 model, slowing tumor growth more than either antibody alone[84].
Most recently described is the interaction between VISTA and syndecan-2, a monocyte proteoglycan[76]. This interaction was identified via CRISPR/Cas9 screenings and demonstrated that heparan sulfate proteoglycan (HSPG) pathway enzymes and syndecan-2 mediate VISTA binding to monocytes[76]. The HSPG pathway is important in monocyte chemotaxis, migration, and maturation[85]. The syndecan-2 antibody prevented the VISTA antibody from binding primary human monocytes, and like the VISTA monoclonal antibodies in clinical trials, this antibody demonstrated the requirement for Fc crosslinking. Interestingly, syndecan-2 is upregulated in some of the same cancers as VISTA, such as colorectal and prostate, as previously discussed[86,87]. This indicates that VISTA antagonism could have an activating effect on monocytes by blocking the interaction between VISTA and syndecan-2 in the TME.
Lastly, VISTA has also been reported to interact with itself via homophilic cis and trans interactions. This occurs in the context of p53-induced VISTA expression, which can lead to signaling-mediated phagocytic clearance of apoptotic cells[77]. However, another group was unable to verify this homotypic interaction of VISTA with itself[74], so this interaction may only occur under particular conditions. Identifying the binding partner(s) of VISTA is challenging due to the lack of functional data to determine whether these interactions are biologically relevant in the TME.
THE LANDSCAPE OF VISTA THERAPEUTICS IN RESEARCH AND DEVELOPMENT
Numerous preclinical and clinical studies are underway to evaluate the activities of VISTA-based therapeutics [Table 2]. There are numerous drugs of interest in preclinical development, including VISTA mAbs PMC-309 by PharmAbcine[88], VTX-0811 by Verseau[89], and KVA12.1 by Kineta[90], SNS-101[91], and INT-18[92].
Current status of VISTA therapeutics in preclinical and clinical development
| Drug | Type | Target | Company | Stage | Cancers |
| JNJ-61610588 | IgG1 mAb | VISTA | Janssen/ImmuNext | Phase I: [D] NCT02671955 | Advanced solid tumors |
| CI-8993 | IgG1 mAb | VISTA | Curis/ImmuNext | Phase I: [IP] NCT04475523 | Relapsed/refractory solid tumors |
| CA-170 | Small molecule | VISTA & PD-L1 | Curis/ImmuNext | Phase I: [C] NCT02812875 | Advanced solid tumors or lymphomas |
| CA-170 | Small molecule | VISTA & PD-L1 | Curis/Aurigene | Phase II: [IP] CTRI/2017/12011026 | Advanced solid tumors or lymphomas |
| K01401-020 W0180 +/- Pembrolizumab | mAb | VISTA +/- PD-1 | Pierre Fabre | Phase Ia, 1b: [ IP] NCT04564417 | Locally advance or metastatic solid tumors |
| HMBD-002 | IgG4 mAb | VISTA | Hummingbird | Phase I/II: [IP] NCT05082610 | Advanced solid tumors |
| KVA 12.1 | IgG1 mAb | VISTA | Kineta | Preclinical | - |
| VTX-0811 | mAb | PSGL-1 | Verseua | Preclinical | - |
| PMC-309 | IgG1 mAb | VISTA | PharmAbcine | Preclinical | - |
| SNS-101 | IgG1 mAb | VISTA/PSGL-1 | Sensei Biotherapeutics | Preclinical | - |
| IMT-18 | mAb | VSIG-3 | iOMx | Preclinical | - |
The first clinical trial (NCT02671955) studying a drug that targeted VISTA involved an antagonistic mAb (JNJ-61610588) developed by ImmuNext in collaboration with Janssen Biotech[93]. This phase I trial enrolled twelve participants with advanced cancers but was terminated by Janssen. The asset was returned to ImmuNext and licensed by Curis. Currently underway is a phase I clinical trial (NCT04475523) using
CA-170 is a small molecule that targets both VISTA and PD-L1, potentially by involving binding to VISTA’s histidine binding sites or forming a defective ternary PD-1/PD-L1 complex[96]. The phase I trial (NCT02812875) was successful[96], and the phase II trial (CTRI/2017/12/011026) demonstrated efficacy[97] but was discontinued. Another clinical trial is evaluating the VISTA mAb W0180 in patients with locally advanced or metastatic solid tumors (NCT04564417)[98]. This phase Ia trial, led by Pierre Fabre, will evaluate the safety of W0180 as a monotherapy, and phase Ib will involve treatment with W0180 and/or pembrolizumab (anti-PD-1). A monoclonal IgG4 anti-VISTA antibody (HMBD-002) is being developed by Hummingbird, with the phase I/II trial ongoing (NCT05082610)[99]. This antibody is cross-reactive to murine VISTA and has been shown to be therapeutically effective in preclinical murine tumor models[100].
FUTURE PERSPECTIVES
Combination ICI therapy approaches have potential to improve cancer patient outcomes. Here, we will discuss the potential for the use of anti-VISTA in conjunction with anti-PD-1 and anti-CTLA-4, based on preclinical murine models, data from patient samples, and current clinical trials. In mice, blockade of PD-L1 and VISTA resulted in 80% tumor regression in the CT26 colon cancer model, which was superior to either treatment alone[28]. A murine RCC model demonstrated that co-blockade of VISTA and PD-1/PD-L1 resulted in a greater reduction of tumor growth relative to either treatment alone[28]. Furthermore, survival is significantly higher in the B16 melanoma model using co-blockade of PD-L1 and VISTA, as compared to monotherapy, despite being a less immunogenic model than CT26. These data demonstrate that VISTA antagonism may be beneficial even in cancers that are less likely to respond to immunotherapy. For example, PDAC is a poorly immunogenic tumor, but the high expression of VISTA on CD68+ TAMs and TCs indicates that this cancer would be a strong candidate for anti-VISTA treatment[40,51].
Based on these data, VISTA activity in the TME can be modulated. VISTA blockade is especially attractive in the context of overcoming adaptive resistance, particularly following the use of other ICIs like anti-PD-1 and anti-CTLA-4. As shown in Table 1, numerous studies have shown upregulation of VISTA in ICs after immune checkpoint blockade. This, combined with the relevance of VISTA expression on TCs and SCs, suggests that VISTA is an attractive therapeutic target.
VISTA blockade may also be used in conjunction with radiation therapy. A murine glioma model comparing the effects of radiation on WT mice versus VISTA KO mice demonstrated that the VISTA KO mice survived significantly longer than the WT[20]. Another study showed that the combination of VISTA and PD-1 co-blockade (with cyclophosphamide) with radiation therapy significantly improves tumor control and overall survival in a mouse model of triple-negative breast cancer[101]. This combination therapy approach overcame some of the mechanisms of adaptive resistance discussed earlier, specifically by depleting MDSCs from the TME, as well as increasing priming and infiltration of tumor specific CTLs to the TME.
It is worth noting that the clinical trials thus far have mostly shown safety and dose-liming toxicities. While the risk for immune-related adverse events in combination therapy is higher than that of monotherapy, preclinical models can be indicative of the extent to which that risk increases. For example, VISTA/PD-1 double KO mice do not develop overt autoimmune disease[28], while CTLA-4 KO mice die very young due to severe inflammation[102,103]. Since the phenotype of the VISTA/PD-1 double KO is relatively benign to that of the CTLA-4 KO mice, co-blockade of PD-1 and VISTA may be more therapeutically attractive relative to the co-blockade of PD-1 and CTLA-4 seen clinically.
CONCLUSION
VISTA modulates both immune cell recruitment to the TME and immune cell function within the TME. It is especially critical to evaluate these subsets in various murine cancer models and in humans, so anti-VISTA therapy can be tailored to specific cancer type, stage, and immunogenicity. The anti-VISTA antibodies in clinical trials may also help shed light on the basic biological mechanisms of VISTA and its potential binding partners. Although detailed studies are required to evaluate the mechanistic role of VISTA across immune cell subsets in the TME, it is apparent that VISTA plays a key regulatory role in the TME, and that VISTA antagonism will be especially beneficial in combination therapy approaches to mitigate adaptive resistance and improve patient outcomes.
DECLARATIONS
Authors’ contributionsWrote the manuscript: Rabadi D, Sajani A
Critically reviewed the content: Rabadi D, Sajani AA, Lines JL, Noelle RJ
Read and approved the final manuscript: Rabadi D, Sajani AA, Lines JL, Noelle RJ
Availability of data and materialsNot available.
Financial support and sponsorshipResearch was supported by NIH grants R01AR070760 and R01CA214062 (Randolph J. Noelle).
Conflicts of interestRandolph J. Noelle is an inventor on patent applications (10035857, 9631018, 9217035, 8501915, 8465740, 8236304, and 8231872) submitted by Dartmouth College, and patent applications (9890215 and 9381244) submitted by Kings College London and Dartmouth College and a co-founder of ImmuNext, a company involved in the development of VISTA-related assets. These applications cover the use of VISTA targeting for modulation of the immune response. Dina Rabadi is a past employee of ImmuNext.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Weir HK, Thompson TD, Stewart SL, White MC. Cancer incidence projections in the united states between 2015 and 2050. Prev Chronic Dis 2021;18:E59.
2. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017;168:707-23.
4. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 2018;8:1069-86.
5. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015;15:486-99.
6. Thommen DS, Schreiner J, Müller P, et al. Progression of lung cancer is associated with increased dysfunction of t cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res 2015;3:1344-55.
7. Xia A, Zhang Y, Xu J, Yin T, Lu XJ. T Cell dysfunction in cancer immunity and immunotherapy. Front Immunol 2019;10:1719.
8. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345-56.
9. Le Mercier I, Chen W, Lines JL, et al. VISTA regulates the development of protective antitumor immunity. Cancer Res 2014;74:1933-44.
10. Kakavand H, Jackett LA, Menzies AM, et al. Negative immune checkpoint regulation by VISTA: a mechanism of acquired resistance to anti-PD-1 therapy in metastatic melanoma patients. Mod Pathol 2017;30:1666-76.
11. Gao J, Ward JF, Pettaway CA, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med 2017;23:551-5.
12. Broughton TWK, ElTanbouly MA, Schaafsma E, et al. Defining the signature of VISTA on myeloid cell chemokine responsiveness. Front Immunol 2019;10:2641.
13. Flies DB, Wang S, Xu H, Chen L. Cutting edge: A monoclonal antibody specific for the programmed death-1 homolog prevents graft-versus-host disease in mouse models. J Immunol 2011;187:1537-41.
14. ElTanbouly MA, Zhao Y, Nowak E, et al. VISTA is a checkpoint regulator for naïve T cell quiescence and peripheral tolerance. Science 2020;367:eaay0524.
15. Wang L, Rubinstein R, Lines JL, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med 2011;208:577-92.
16. Mehta N, Maddineni S, Mathews II, Sperberg AP, Huang PS, Cochran JR. Structure and functional binding epitope of v-domain Ig suppressor of t-cell activation (VISTA). Cell Rep 2019;28:2509-16.e5.
17. Slater BT, Han X, Chen L, Xiong Y. Structural insight into T cell coinhibition by PD-1H (VISTA). Proc Natl Acad Sci USA 2020;117:1648-57.
18. Yum JI, Hong YK. Terminating cancer by blocking VISTA as a novel immunotherapy: hasta la vista, baby. Front Oncol 2021;11:658488.
19. Lines JL, Pantazi E, Mak J, et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res 2014;74:1924-32.
20. Flies DB, Han X, Higuchi T, et al. Coinhibitory receptor PD-1H preferentially suppresses CD4+ T cell-mediated immunity. J Clin Invest 2014;124:1966-75.
21. Tanbouly MA, Croteau W, Noelle RJ, Lines JL. VISTA: a novel immunotherapy target for normalizing innate and adaptive immunity. Semin Immunol 2019;42:101308.
22. El Tanbouly MA, Schaafsma E, Smits NC, et al. VISTA Re-programs macrophage biology through the combined regulation of tolerance and anti-inflammatory pathways. Front Immunol 2020;11:580187.
23. Wang L, Le Mercier I, Putra J, et al. Disruption of the immune-checkpoint VISTA gene imparts a proinflammatory phenotype with predisposition to the development of autoimmunity. Proc Natl Acad Sci USA 2014;111:14846-51.
24. Ceeraz S, Sergent PA, Plummer SF, et al. VISTA deficiency accelerates the development of fatal murine lupus nephritis. Arthritis Rheumatol 2017;69:814-25.
25. Sergent PA, Plummer SF, Pettus J, et al. Blocking the VISTA pathway enhances disease progression in (NZB × NZW) F1 female mice. Lupus 2018;27:210-6.
26. Li N, Xu W, Yuan Y, et al. Immune-checkpoint protein VISTA critically regulates the IL-23/IL-17 inflammatory axis. Sci Rep 2017;7:1485.
27. Flies DB, Higuchi T, Chen L. Mechanistic assessment of PD-1H coinhibitory receptor-induced T Cell tolerance to allogeneic antigens. J Immunol 2015;194:5294-304.
28. Liu J, Yuan Y, Chen W, et al. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc Natl Acad Sci USA 2015;112:6682-7.
29. Shrestha R, Prithviraj P, Anaka M, et al. Monitoring immune checkpoint regulators as predictive biomarkers in hepatocellular carcinoma. Front Oncol 2018;8:269.
30. Zhang M, Pang HJ, Zhao W, et al. VISTA expression associated with CD8 confers a favorable immune microenvironment and better overall survival in hepatocellular carcinoma. BMC Cancer 2018;18:511.
31. Böger C, Behrens HM, Krüger S, Röcken C. The novel negative checkpoint regulator VISTA is expressed in gastric carcinoma and associated with PD-L1/PD-1: A future perspective for a combined gastric cancer therapy? Oncoimmunology 2017;6:e1293215.
32. Schoop H, Bregenzer A, Halske C, et al. Therapy resistance in neoadjuvantly treated gastric cancer and cancer of the gastroesophageal junction is associated with an increased expression of immune checkpoint inhibitors-comparison against a therapy naïve cohort. Transl Oncol 2020;13:165-76.
33. Villarroel-Espindola F, Yu X, Datar I, et al. Spatially resolved and quantitative analysis of VISTA/PD-1H as a novel immunotherapy target in human non-small cell lung cancer. Clin Cancer Res 2018;24:1562-73.
34. Liao H, Zhu H, Liu S, Wang H. Expression of V-domain immunoglobulin suppressor of T cell activation is associated with the advanced stage and presence of lymph node metastasis in ovarian cancer. Oncol Lett 2018;16:3465-72.
35. Mulati K, Hamanishi J, Matsumura N, et al. VISTA expressed in tumour cells regulates T cell function. Br J Cancer 2019;120:115-27.
36. Zong L, Zhang M, Wang W, Wan X, Yang J, Xiang Y. PD-L1, B7-H3 and VISTA are highly expressed in gestational trophoblastic neoplasia. Histopathology 2019;75:421-30.
37. Kuklinski LF, Yan S, Li Z, et al. VISTA expression on tumor-infiltrating inflammatory cells in primary cutaneous melanoma correlates with poor disease-specific survival. Cancer Immunol Immunother 2018;67:1113-21.
38. Choi JW, Kim YJ, Yun KA, et al. The prognostic significance of VISTA and CD33-positive myeloid cells in cutaneous melanoma and their relationship with PD-1 expression. Sci Rep 2020;10:14372.
39. Wu L, Deng WW, Huang CF, et al. Expression of VISTA correlated with immunosuppression and synergized with CD8 to predict survival in human oral squamous cell carcinoma. Cancer Immunol Immunother 2017;66:627-36.
40. Hou Z, Pan Y, Fei Q, et al. Prognostic significance and therapeutic potential of the immune checkpoint VISTA in pancreatic cancer. J Cancer Res Clin Oncol 2021;147:517-31.
41. Ghouzlani A, Lakhdar A, Rafii S, Karkouri M, Badou A. The immune checkpoint VISTA exhibits high expression levels in human gliomas and associates with a poor prognosis. Sci Rep 2021;11:21504.
42. He XL, Zhou Y, Lu HZ, Li QX, Wang Z. Prognostic value of VISTA in solid tumours: a systematic review and meta-analysis. Sci Rep 2020;10:2662.
43. Loeser H, Kraemer M, Gebauer F, et al. The expression of the immune checkpoint regulator VISTA correlates with improved overall survival in pT1/2 tumor stages in esophageal adenocarcinoma. Oncoimmunology 2019;8:e1581546.
44. Cao X, Ren X, Zhou Y, et al. VISTA expression on immune cells correlates with favorable prognosis in patients with triple-negative breast cancer. Front Oncol 2020;10:583966.
45. Hernandez-Martinez JM, Vergara E, Zatarain-Barrón ZL, Barrón-Barrón F, Arrieta O. VISTA/PD-1H: a potential target for non-small cell lung cancer immunotherapy. J Thorac Dis 2018;10:6378-82.
46. Zong L, Zhou Y, Zhang M, Chen J, Xiang Y. VISTA expression is associated with a favorable prognosis in patients with high-grade serous ovarian cancer. Cancer Immunol Immunother 2020;69:33-42.
47. Xie S, Huang J, Qiao Q, et al. Expression of the inhibitory B7 family molecule VISTA in human colorectal carcinoma tumors. Cancer Immunol Immunother 2018;67:1685-94.
48. Kuang L, He Y. Potential value of V-domain Ig suppressor of T-cell activation for assessing progn osis in cervical cancer and as a target for therapy. Int J Clin Exp Pathol 2020;13:26-37.
49. Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 2018;24:541-50.
50. Érsek B, Silló P, Cakir U, et al. Melanoma-associated fibroblasts impair CD8+ T cell function and modify expression of immune checkpoint regulators via increased arginase activity. Cell Mol Life Sci 2021;78:661-73.
51. Blando J, Sharma A, Higa MG, et al. Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer. Proc Natl Acad Sci USA 2019;116:1692-7.
52. Wang X, Teng F, Kong L, Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther 2016;9:5023-39.
53. Kondo Y, Ohno T, Nishii N, Harada K, Yagita H, Azuma M. Differential contribution of three immune checkpoint (VISTA, CTLA-4, PD-1) pathways to antitumor responses against squamous cell carcinoma. Oral Oncol 2016;57:54-60.
54. Hong S, Yuan Q, Xia H, et al. Analysis of VISTA expression and function in renal cell carcinoma highlights VISTA as a potential target for immunotherapy. Protein Cell 2019;10:840-5.
55. Jin J, Lin J, Xu A, et al. CCL2: An important mediator between tumor cells and host cells in tumor microenvironment. Front Oncol 2021;11:722916.
56. Weber R, Fleming V, Hu X, et al. Myeloid-derived suppressor cells hinder the anti-cancer activity of immune checkpoint inhibitors. Front Immunol 2018;9:1310.
57. Clavijo PE, Moore EC, Chen J, et al. Resistance to CTLA-4 checkpoint inhibition reversed through selective elimination of granulocytic myeloid cells. Oncotarget 2017;8:55804-20.
58. Deng J, Li J, Sarde A, et al. Hypoxia-induced VISTA promotes the suppressive function of myeloid-derived suppressor cells in the tumor microenvironment. Cancer Immunol Res 2019;7:1079-90.
59. Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY, Mou XZ. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol 2020;353:104119.
60. Ceeraz S, Eszterhas SK, Sergent PA, et al. VISTA deficiency attenuates antibody-induced arthritis and alters macrophage gene expression in response to simulated immune complexes. Arthritis Res Ther 2017;19:270.
61. Veglia F, Gabrilovich DI. Dendritic cells in cancer: the role revisited. Curr Opin Immunol 2017;45:43-51.
62. Wang L, Jia B, Claxton DF, et al. VISTA is highly expressed on MDSCs and mediates an inhibition of T cell response in patients with AML. Oncoimmunology 2018;7:e1469594.
63. Xu W, Dong J, Zheng Y, et al. Immune-checkpoint protein VISTA regulates antitumor immunity by controlling myeloid cell-mediated inflammation and immunosuppression. Cancer Immunol Res 2019;7:1497-510.
64. Ohno T, Zhang C, Kondo Y, et al. The immune checkpoint molecule VISTA regulates allergen-specific Th2-mediated immune responses. Int Immunol 2018;30:3-11.
66. Liu H, Li X, Hu L, et al. A crucial role of the PD-1H coinhibitory receptor in suppressing experimental asthma. Cell Mol Immunol 2018;15:838-45.
67. ElTanbouly MA, Zhao Y, Schaafsma E, et al. VISTA: a target to manage the innate cytokine storm. Front Immunol 2020;11:595950.
68. Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The Nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol 2016;37:208-20.
69. Law AMK, Valdes-Mora F, Gallego-Ortega D. Myeloid-derived suppressor cells as a therapeutic target for cancer. Cells 2020;9:561.
70. Wang Q, He J, Flies DB, Luo L, Chen L. Programmed death one homolog maintains the pool size of regulatory T cells by promoting their differentiation and stability. Sci Rep 2017;7:6086.
71. Wei SC, Anang NAS, Sharma R, et al. Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc Natl Acad Sci USA 2019;116:22699-709.
72. Im SJ, Ha SJ. Re-defining T-Cell exhaustion: subset, function, and regulation. Immune Netw 2020;20:e2.
73. Miller BC, Sen DR, Al Abosy R, et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol 2019;20:326-36.
74. Johnston RJ, Su LJ, Pinckney J, et al. VISTA is an acidic pH-selective ligand for PSGL-1. Nature 2019;574:565-70.
75. Wang J, Wu G, Manick B, et al. VSIG-3 as a ligand of VISTA inhibits human T-cell function. Immunology 2019;156:74-85.
76. Rogers BM, Smith L, Dezso Z, et al. VISTA is an activating receptor in human monocytes. J Exp Med 2021;218:e20201601.
77. Yoon KW, Byun S, Kwon E, et al. Control of signaling-mediated clearance of apoptotic cells by the tumor suppressor p53. Science 2015;349:1261669.
78. Carlow DA, Gossens K, Naus S, Veerman KM, Seo W, Ziltener HJ. PSGL-1 function in immunity and steady state homeostasis. Immunol Rev 2009;230:75-96.
79. Tinoco R, Otero DC, Takahashi AA, Bradley LM. PSGL-1: a new player in the Immune checkpoint landscape. Trends Immunol 2017;38:323-35.
80. Matsumoto M, Miyasaka M, Hirata T. P-selectin glycoprotein ligand-1 negatively regulates T-cell immune responses. J Immunol 2009;183:7204-11.
81. Wu H, Estrella V, Beatty M, et al. T-cells produce acidic niches in lymph nodes to suppress their own effector functions. Nat Commun 2020;11:4113.
82. Molloy MGY, Rothstein J. InventorIdentification of vsig8 as the putative vista receptor and its use thereof to produce vista/vsig8 modulators2016.
83. VSIG8 the Human Protein Atlas. Available from: https://www.proteinatlas.org/ENSG00000243284-VSIG8 [Last accessed on 15 June 2022].
84. Mehta N, Maddineni S, Kelly RL, et al. An engineered antibody binds a distinct epitope and is a potent inhibitor of murine and human VISTA. Sci Rep 2020;10:15171.
85. Farrugia BL, Lord MS, Melrose J, Whitelock JM. The role of heparan sulfate in inflammation, and the development of biomimetics as anti-inflammatory strategies. J Histochem Cytochem 2018;66:321-36.
86. Hua R, Yu J, Yan X, et al. Syndecan-2 in colorectal cancer plays oncogenic role via epithelial-mesenchymal transition and MAPK pathway. Biomed Pharmacother 2020;121:109630.
87. Popović A, Demirović A, Spajić B, Stimac G, Kruslin B, Tomas D. Expression and prognostic role of syndecan-2 in prostate cancer. Prostate Cancer Prostatic Dis 2010;13:78-82.
88. Park CH, Byun SS, An JY, et al. Abstract 1626: PMC309, a highly selective anti-VISTA antibody enhances T cell activation through blocking the interaction of T cells and myeloid derived suppressor cells (MDSC). Cancer Res 2021;81:1626-1626.
89. ElTanbouly MA, Schaafsma E, Noelle RJ, Lines JL. VISTA: coming of age as a multi-lineage immune checkpoint. Clin Exp Immunol 2020;200:120-30.
90. Guillaudeux T, Iadonato S, Tarcha E, Philips C. 268 KVA 12.1 a novel fully human anti-VISTA antibody: clinical trial design in monotherapy and in combination with an anti-PD1 antibody. J ImmunoTherapy Cancer 2021;9:A1-A1054.
91. Thisted T, Mukherjee A, Malhotra K, Biesova Z, Kleschenko Y, Jiang ZG, et al. 228 Antagonistic pH-selective VISTA antibody SNS-101 potentiates anti-PD-1/PD-L1-induced anti-tumor immunity. J ImmunoTherapy Cancer 2021; doi: 10.1136/jitc-2021-sitc2021.228.
92. Pipeline iOmx therapeutics. Available from: https://iomx.com/pipeline [Last accessed on 15 June 2022].
93. A study of safety, pharmacokinetics, pharmacodynamics of JNJ-61610588 in participants with advanced cancer. Available from: https://ClinicalTrials.gov/show/NCT02671955 [Last accessed on 15 June 2022].
94. Phase 1 study of CI-8993 anti-VISTA antibody in patients with advanced solid tumor malignancies. Available from: https://ClinicalTrials.gov/show/NCT04475523 [Last accessed on 15 June 2022].
95. Scott F, Wichmann C, Burvenich I, McDonald A, Guo N, Rigopoulos A, et al. 324 Preclinical evaluation of anti-VISTA antibody CI-8993 in a syngeneic huVISTA-KI model. J ImmunoTherapy Cancer 2021; doi: 10.1136/jitc-2021-sitc2021.324.
96. Sasikumar PG, Sudarshan NS, Adurthi S, et al. PD-1 derived CA-170 is an oral immune checkpoint inhibitor that exhibits preclinical anti-tumor efficacy. Commun Biol 2021;4:699.
97. Radhakrishnan V, Bakhshi S, Prabhash K, Deshmukh C, Nag S, Lakshmiah KC, et al. Abstract P714: phase 2 trial of CA-170, a novel oral small molecule dual inhibitor of immune checkpoints VISTA and PD-1, in patients (pts) with advanced solid tumor and Hodgkin lymphoma2018.
98. First-In-Human (FIH) Study of W0180 as single agent and in combination with pembrolizumab in adults with locally advanced or metastatic solid tumors. Available from: https://ClinicalTrials.gov/show/NCT04564417 [Last accessed on 15 June 2022].
99. A study of HMBD-002, a monoclonal antibody targeting VISTA, as monotherapy and combined with pembrolizumab. Available from: https://ClinicalTrials.gov/show/NCT05082610 [Last accessed on 15 June 2022].
100. Thakkar D, Paliwal S, Dharmadhikari B, et al. Rationally targeted anti-VISTA antibody that blockades the C-C' loop region can reverse VISTA immune suppression and remodel the immune microenvironment to potently inhibit tumor growth in an Fc independent manner. J Immunother Cancer 2022;10:e003382.
101. Pilones KA, Hensler M, Daviaud C, et al. Converging focal radiation and immunotherapy in a preclinical model of triple negative breast cancer: contribution of VISTA blockade. Oncoimmunology 2020;9:1830524.
102. Tivol EA, Borriello F, Schweitzer A, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995;3:541-7.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Rabadi D, Sajani AA, Noelle RJ, Lines JL. The role of VISTA in the tumor microenvironment. J Cancer Metastasis Treat 2022;8:24. http://dx.doi.org/10.20517/2394-4722.2022.06
AMA Style
Rabadi D, Sajani AA, Noelle RJ, Lines JL. The role of VISTA in the tumor microenvironment. Journal of Cancer Metastasis and Treatment. 2022; 8(5): 24. http://dx.doi.org/10.20517/2394-4722.2022.06
Chicago/Turabian Style
Rabadi, Dina, Alia A. Sajani, Randolph J. Noelle, J. Louise Lines. 2022. "The role of VISTA in the tumor microenvironment" Journal of Cancer Metastasis and Treatment. 8, no.5: 24. http://dx.doi.org/10.20517/2394-4722.2022.06
ACS Style
Rabadi, D.; Sajani AA.; Noelle RJ.; Lines JL. The role of VISTA in the tumor microenvironment. J. Cancer. Metastasis. Treat. 2022, 8, 24. http://dx.doi.org/10.20517/2394-4722.2022.06
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 11 clicks
Cite This Article 11 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.