Current and future opportunities for liquid biopsy of circulating biomarkers to aid in early cancer detection
Abstract
Early diagnosis of cancer can significantly improve treatment and survival outcomes. Imaging and tissue biopsy are the gold standard diagnostic approaches but are costly, invasive, and often unable to detect early-stage tumors. The past decade has marked an acceleration in the discovery and development of liquid biopsy tests for aiding in the detection of various types of tumor markers in non-tissue samples, such as blood. Liquid biopsy markers include circulating tumor cells, as well as tumor cell fragments, nucleic acids, and proteins. Liquid biopsy may be useful in screening patients considered to be at high risk of developing cancer, for refining diagnosis when combined with other test results, and for early detection of recurrence. Advances in big data analytics, informatics, and artificial intelligence will make it possible to combine patient history, clinical data, and liquid biopsy marker profiles to achieve more accurate and earlier diagnosis. In this review, we summarize the current use of liquid biopsy in cancer care, including the development of multi-analyte panels to improve diagnostic accuracy and detect several cancer types in a single assay. We highlight recent advances for potential future applications of liquid biopsy to aid in the diagnosis of early-stage lung cancer. We also discuss the opportunities and challenges of integrating liquid biopsy into current algorithms for cancer screening and diagnosis.
Keywords
INTRODUCTION
Cancer is the leading cause of death worldwide, second only to cardiovascular diseases; the lifetime risk of developing cancer is nearly 40% and accounts for approximately 20% of deaths in the United States[1]. Most cancer diagnoses continue to be made at advanced stages and are associated with poor prognoses; this is in part due to a lack of effective cancer screening programs. As early-stage cancer treatments are generally more effective and less invasive, the diagnosis of cancer at earlier stages would significantly improve treatment and survival outcomes for many patients[2].
Gold standard diagnostic testing for solid tumors involves imaging combined with pathology-based assessments of biopsied tissues and other biological samples from or near the suspected malignant lesions. Tumor tissue presence and stage of progression are evaluated based on a combination of morphological and histological markers in the biopsied tissues. However, tissue biopsy is invasive and associated with significant co-morbidities, including potential infection, pain, and bleeding. While diagnostic imaging is less invasive, it is costly and the ability to detect early-stage tumors is limited. Some imaging modalities also involve exposure to radiation, which limits their utility for a serial follow-up to detect recurrence. To address these issues, the past decade has marked an acceleration in the discovery and development of minimally invasive tests for tumor markers in easily collected patient samples, such as blood, which would require only routine venipuncture or fingerstick.
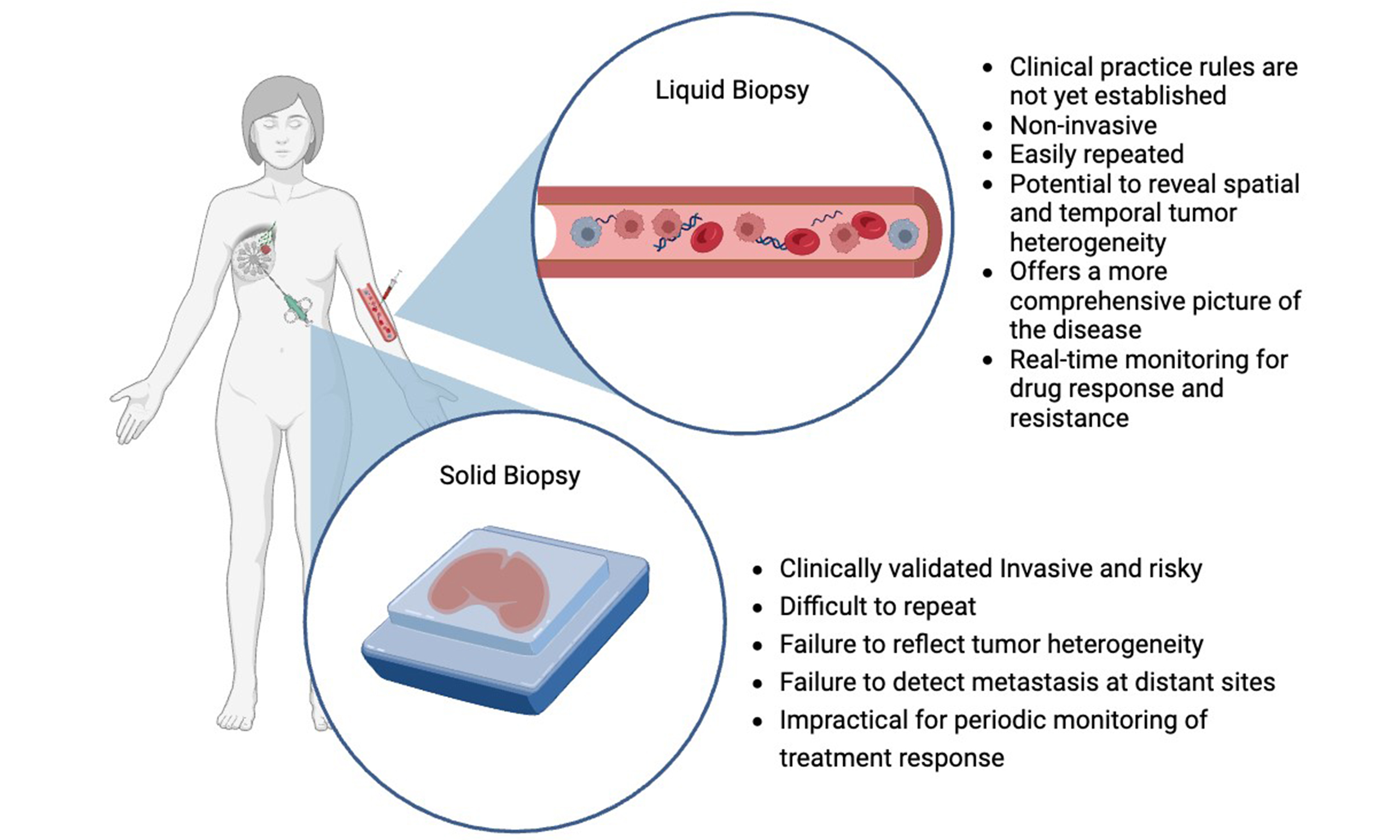
The term “liquid biopsy” refers to the sampling and analysis of any non-solid biological materials sourced from patients (e.g., blood, urine, saliva)[3]. Similar to tissue biopsy, the goal of liquid biopsy is to gather information about a potential lesion or a tumor that will inform a patient’s clinical care journey [Figure 1]. A particular advantage of liquid biopsy may be to aid in the earlier detection of cancers, prior to the development of tumors and/or lesions large enough to detect by imaging or tissue biopsy. Liquid biopsy may also help inform differential diagnosis through molecular phenotyping of tumors and allow for the earlier detection of recurrent disease. In this review, we summarize the current use of liquid biopsy to detect various types of tumor markers and their potential application in the early diagnosis of cancer. We highlight the use of protein-based tumor markers in combination with other markers [e.g., circulating tumor cells (CTCs), cell-free circulating tumor DNA (ctDNA)] into multi-analyte panels, as well as the integration of liquid biopsy into comprehensive algorithms for cancer screening and diagnosis. The current advancement of big data analytics, informatics, and artificial intelligence can be used to combine clinical data and liquid biopsy results for more accurate diagnosis and tumor profiling[4,5]. Finally, we discuss recent advances in the application of liquid biopsy in the oncology field.
LIQUID BIOPSY FOR CANCER DIAGNOSIS
Types of cancer biomarkers utilized in liquid biopsy
Liquid biopsies can be used to test for various types of diagnostic, prognostic, or predictive tumor markers, including CTCs and tumor cell fragments released into a patient’s circulation, ctDNA, and tumor cell proteins and secreted factors [Figure 2][6]. Recent liquid biopsy research efforts have focused extensively on the early detection of CTCs and on nucleic acid interrogations, including deep sequencing of ctDNA[3,7]. For the last three decades, a large body of work has also identified various circulating protein-based tumor-associated biomarkers and their utility in liquid biopsy for noninvasive cancer screening and aiding in diagnosis [Table 1]. These markers can help identify patients who are likely to have a malignancy and require further diagnostic workup. Recent research has also described the potential use of liquid biopsy to detect protein markers within extracellular vesicles derived from breast cancer cells[8] and keratins as candidate protein biomarkers[9].
Figure 2. Tumor protein biomarkers of various cancer types. AFP: Alpha-fetoprotein; B2M: beta 2-microglobulin; β-hCG: beta-human chorionic gonadotropin; CA: cancer antigen; CA 19-9: carbohydrate antigen 19-9; CEA: carcinoembryonic antigen; Cyfra 21-1: cytokeratin 19 fragments; HE4: human epididymis secretory protein 4; Ig: immunoglobulin; LG2m: laminin γ2 monomer; NMP22: nuclear matrix protein-22; NSE: neuron-specific enolase; PIVKA-II: protein induced by vitamin K absence-II; ProGRP: pro-gastrin-releasing peptide; PSA: prostate-specific antigen; SCCA: squamous cell carcinoma antigen; Tg: thyroglobulin.
Clinical indications for circulating protein-based tumor markers in blood-based liquid biopsies[27,45-47]
| Clinical indication | Protein tumor marker examples |
| Differential diagnosis | • ProGRP is indicative of small cell lung cancer phenotype • SCCA is indicative of NSCLC squamous cell carcinoma phenotype |
| Prognosis and staging | • AFP association and progression of HCC • Free and total PSA for prostate cancer |
| Cancer monitoring | • CEA for monitoring colorectal, gastric, breast, lung, prostate, pancreatic, and ovarian carcinomas • CA 72-4 for monitoring upper GI and mucinous ovarian cancers |
| Treatment prediction | • CEA and Cyfra 21-1 signature predictive of improved survival in advanced NSCLC patients on linifanib treatment in an exploratory study |
Improving cancer diagnosis with liquid biopsy multi-analyte panels
Historically, protein-based tumor markers such as carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA 19-9), and cancer antigen 125 (CA 125) have been used to monitor cancer progression or recurrence in previously diagnosed patients. While these protein biomarkers may be useful for follow-up of patients with known diseases, none have been approved for screening or to aid in diagnosis, in part because of their low clinical sensitivity or specificity[10-12]. A recent trend to combat this limitation is to combine several tumor markers, which may include CTCs, proteins, and nucleic acid-based markers such as ctDNA or methylated DNA, into multi-analyte panels that provide a more accurate composite result or risk score [Table 2]. While individual tumor marker panel results alone are not sufficient for providing a definitive diagnosis, they can be included in algorithms with other factors to provide an initial assessment of the likelihood of malignancy and identify patients who need more extensive follow-up with more intensive diagnostic procedures.
Examples of multi-analyte tumor marker tests to aid in early detection of cancers (approved or under clinical development)
| Test indication & clinical utility | Test description | References |
| Multi-analyte panels of nucleic acid-based markers | ||
| Multicancer early detection test (CancerSEEK) | CancerSEEK is in clinical development. It detects 8 common cancers at early stages. CancerSEEK utilizes multiplexed PCR ctDNA test for mutations with protein biomarker tests to aid in detecting tumors, including 5 types for which there is currently no clinically available screening test. Clinical Investigators & Thrive Corporation have received FDA breakthrough device designation | Lennon et al., 2020[11] Cohen et al., 2019[20] |
| Galleri multicancer early detection test | This NGS-based test measures ctDNA methylation patterns to aid in detection of many solid tumors. Clinical investigators & Grail Corporation have FDA breakthrough device designation | Liu et al., 2020[40] |
| Epi proColon test for aiding in early detection of CRC | Real-time PCR test detects methylated cytosines in the SEPTIN9 gene for detection of CRC. Epigenomics Corporation’s test was FDA-approved in 2016 and is in clinical use | Lamb et al., 2017[48] |
| Cologuard test for early detection of CRC | Identifies DNA mutations and altered methylation in stool (FIT), associated with the possibility of colon cancer or precancer. Cologuard was FDA-approved for clinical use in 2015 | Imperiale et al., 2014[49] |
| Bluestar test for detection of pancreatic cancer | Detects 5-hydroxymethylcytosine alterations in cell-free DNA of genes involved in pancreatic cancer | Guler et al., 2020[50] |
| Multi-analyte panels that include protein-based markers | ||
| EarlyCDT-Lung test for aiding in early detection of lung cancer | The EarlyCDT-Lung test from Oncoimmune Holdings detects 7 autoantibodies and was approved in the EU in 2018 | Macdonald et al., 2017[51] |
| Freenome multi-omic test for early detection of CRC | Freenome’s in-research multi-analyte test measures a combination of cell-free nucleic acids and plasma protein-based markers to more accurately detect CRC | Kleif et al., 2020[22] Wan et al., 2019[41] |
| Liquid biopsy for detection of early-stage HCC | Two independent groups are developing assays that combine ctDNA methylation signatures and protein-based markers AFP and DCP | Cai et al., 2021[16] Guo et al., 2021[17] |
| Incorporation of protein-based markers into clinical algorithms | ||
| Emerging algorithms for early detection of HCC | ASAP, Random Forest, and GALAD algorithms are in clinical development that combine patient factors such as age and sex with biomarkers including AFP, DCP, PIVKA-II, and AFP-L3 | Hemken et al., 2019[52] Yang et al., 2019[53] Yang et al., 2019[54] |
The combination of multiple markers can increase diagnostic accuracy by reducing the number of false positives and false negatives[13,14]. Several studies have shown that the clinical sensitivity and specificity of CTC and ctDNA tests can be considerably improved when coupled with protein-based markers for several types of tumors. In a recent proof-of-concept study presented at the 2021 American Association for Cancer Research (AACR) Conference on Pancreatic Cancer, Hsu et al. reported that an assay for both cell-free DNA methylation and CA 19-9 protein in plasma samples had significantly greater sensitivity and specificity for detecting pancreatic ductal adenocarcinoma than either marker alone, even for stage II disease[15]. Another recent study demonstrated the performance of a liquid biopsy assay that detects ctDNA methylation and protein-based biomarkers for diagnosis and prognosis in patients with hepatocellular carcinoma (HCC)[16]. A liquid biopsy assay for liver cancer that combines two ctDNA methylation markers and two protein-based markers [alpha-fetoprotein (AFP) and des-gamma-carboxyprothrombin (DCP)] had a sensitivity of 96.6% for HCC and 86.2% for intrahepatic cholangiocarcinoma (ICC) and high sensitivity for detecting early-stage HCC[17].
The combination of multiple biomarkers into a screening panel also has implications for detecting multiple cancer types simultaneously. A test combining ctDNA with CEA for colorectal cancer (CRC) and pancreatic carcinoma, ctDNA with prostate-specific antigen (PSA), prostate-specific antigen for prostate cancer, and ctDNA with microsatellite alterations for lung cancer, holds promise for significantly earlier diagnosis of all three cancer types[18,19]. Cohen et al. recently described the combination of blood tests for KRAS gene mutations with eight protein-based tumor markers with clear thresholds[20]. The resulting multi-analyte test–CancerSEEK–was superior to any single marker test for early detection of ovarian, liver, stomach, pancreas, and esophageal cancer. They also found that the addition of protein biomarkers to KRAS enhanced the ability to identify the tissue site of origin of early-stage tumors.
INCLUSION OF LIQUID BIOPSY IN DIAGNOSTIC ALGORITHMS
Liquid biopsy for cancer biomarkers can be integrated into existing diagnostic algorithms that consider patient history, clinical factors, and radiologic or imaging results. For example, assays for the protein-based tumor marker PSA and other kallikreins have been combined with other clinical information such as digital rectal examination (DRE) results for prostate cancer screening in high-risk populations[21]. A patient who is considered high-risk based on initial clinical tests such as DRE, demographic factors [e.g., age, sex, body mass index (BMI)], or family history (e.g., genetic markers) may be a candidate for a liquid biopsy test. Patients with a positive test result would likely continue on to additional testing, for example, imaging or tissue biopsy; those with negative results may proceed to watchful waiting with follow-up testing. Another example of a clinically approved algorithm is the risk of malignancy algorithm (ROMA), which utilizes menopausal status and tumor marker concentrations [ovarian tumor markers CA 125 and human epididymis secretory protein 4 (HE4)] to assess malignancy risk in women with pelvic masses and identify those at high risk who should be referred for additional testing to make a definitive diagnosis. The integration of liquid biopsy into diagnostic algorithms may not only help reduce unnecessary imaging, but also shift the point at which a diagnostic decision can be made by identifying patients with cancer at earlier stages, with better prognoses, and quickly providing them with further testing or treatment.
Liquid biopsy could also be used to confirm or refine an initial imaging-based diagnosis. Taking lung cancer as an example, low-dose computed tomography (LDCT) is a highly sensitive imaging test for detecting cancer but has a high false positive rate (poor clinical specificity). Adding liquid biopsy to imaging results could provide additional information to help distinguish between early-stage lung cancer and benign nodules in high-risk patients with indeterminate pulmonary nodules identified by imaging.
The addition of liquid biopsy to diagnostic algorithms is expected to improve clinical decision making; however, several challenges need to be considered before blood-based cancer biomarkers can be incorporated into care. For example, a positive liquid biopsy result may not provide information about the location of the primary lesion, requiring patients to undergo additional testing. A positive liquid biopsy result may indicate the presence of cancer before a lesion is visible on imaging; conversely, patients with a negative result may be directed to watchful waiting. All of these scenarios can create significant concern and worry for patients and clinicians. Diagnostic algorithms, particularly the plan for follow-up assessment after liquid biopsy results, need to perform well in terms of accurately identifying the primary lesion or eliminating patients without disease after further workup[22].
Leveraging big data to integrate liquid biopsy into cancer care
The inclusion of liquid biopsy into cancer diagnostic algorithms will require advanced methods that can integrate various types of data, including results from liquid biopsies, radiomic data, other immunoassays and clinical chemistry assays, demographic information, and family history. These data may be collected at a single point in time or serially, or by different providers along the care pathway, adding to the complexity of data integration and analysis. Advances in digital health, defined as the convergence of digital technologies with healthcare, may accelerate the translation of complex cancer diagnostic algorithms to patient care by consolidating various types of patient data to inform clinical decision making.
APPLICATION OF LIQUID BIOPSY TO POPULATION CANCER SCREENING
In many countries, including Japan, China, and Korea, tumor marker panels are now routinely offered by clinics, along with other preventative health assessments, at annual “wellness checks” to help with early identification of cancer[23]. This application of liquid biopsy to cancer screening in the general population raises concerns about the frequency of false-positive results, which can lead to unnecessary further testing or invasive procedures[24]. The clinical utility of liquid biopsy for cancer screening could be improved through the application of machine learning-based approaches to optimize the interpretation of multi-analyte screening results[25,26]. The current literature lacks objective published data supporting the effectiveness or potential economic impact of including multi-analyte cancer biomarker panels in wellness checks; more studies are needed to determine the best way to integrate liquid biopsy into general health screening programs.
USE OF LIQUID BIOPSY TO DETECT CANCER RECURRENCE
Early clinical studies have demonstrated that liquid biopsy can detect molecular residual disease as well as recurrent disease that is not yet evident by imaging. The protein markers CEA, CA 19-9, CA 125, and CA 15-3 are used to monitor progression or postoperative recurrence for several cancer types[27,28]. As in screening, liquid biopsy could be used to monitor patients following surgery or other local-regional therapy to detect residual disease prior to imaging. Patients with a positive liquid biopsy test may then be referred to advanced imaging with sensitive modalities such as positron emission tomography/computed tomography (PET/CT) to localize residual or metastatic disease. Large clinical studies are needed to determine if serial monitoring using liquid biopsy can replace routine imaging surveillance in patients without evidence of molecular residual disease.
APPLICATION OF LIQUID BIOPSY IN LUNG CANCER
Lung cancer is the deadliest cancer worldwide, accounting for almost 20% of cancer-related fatalities. Lung tumors are typically asymptomatic in their early stages and are often diagnosed at later stages with poorer prognoses, resulting in low treatment success. Considering that the 5-year survival for patients with stage IA lung cancer is greater than 70%, advances in early detection are crucial to enable timely curative surgery[29]. LDCT is currently used for lung cancer screening in patients considered to be at high risk based on various factors and family history. The feasibility of LDCT-based screening is limited by cost and availability, however, making liquid biopsy an exciting possible alternative or complementary approach to detect early-stage lung cancer.
Higher levels of ctDNA have been shown to correlate with worse prognosis for lung cancer[30-32]. ctDNA levels are also associated with tumor volume as measured with CT and PET/CT[33] and may help distinguish between residual disease and treatment-related changes, as well as provide earlier treatment response assessment compared with radiographic approaches[34,35]. Several studies are currently investigating the value of combining noninvasive biomarker tests for risk stratification with LDCT to improve the cost-benefit ratio of screening and possibly reduce mortality. MicroRNAs (miRNAs) are small non-coding RNAs that have been associated with a variety of physiological processes and diseases including cancer. A recent analysis of 84 patients with lung cancer identified in large LDCT screening cohorts[36] utilized a miRNA signature classifier (MSC) risk profile to stratify patients according to their survival. The MSC risk profile was able to refine prognosis within specific clinicopathological groups: 20 out of 24 (83%) patients who died were classified as high-risk based on the MSC test, as were 63% of stage II-IV patients. Conversely, patients classified as having low or intermediate risk by the MSC test had mostly stage I disease (71%) with better survival rates. MSC testing could be integrated into patient care algorithms prior to LDCT to reduce unnecessary imaging in those found to be at low risk.
RECENT ADVANCES IN LIQUID BIOPSY OF CANCER BIOMARKERS
The potential benefits of liquid biopsy for cancer screening and aiding in cancer diagnosis have led to the development of several new multi-analyte tests that can detect multiple cancers simultaneously[37,38]. CancerSEEK, as previously mentioned, detects a combination of 16 ctDNA mutations and 8 protein markers associated with various types of cancer in blood specimens[20]. The diagnostic accuracy of CancerSEEK was assessed in a large study of more than 10,000 women with no history of cancer[39]. Women with positive tests underwent PET imaging to confirm the diagnosis and localize the tumor if possible. CancerSEEK detected 26 of 96 incident cancers during the 12-month study; of these, 15 also had a positive PET result. Nine women underwent surgery based on the combined results of the blood test and imaging results. The test detected cancers in 10 different organs, many of which have no available screening test. In 22 patients who developed lung cancer, 9 were detected by the blood test, and 3 were detected by LDCT. The study authors concluded that CancerSEEK and liquid biopsy, combined with imaging, hold promise for screening and detection of early-stage cancer, but require further optimization to improve the positive predictive value.
Another liquid biopsy assay, Galleri from GRAIL, measures methylated ctDNA markers in more than 50 types of cancer. A recent study of almost 6700 individuals with and without cancer reported 67% sensitivity for detecting a set of 12 cancer types with the highest mortality in the US and 44% sensitivity for detecting all cancer types[40]. The utility of the assay as a screening tool is currently being tested in the SUMMIT study of 13,000 adults with no known cancer diagnosis (NCT03934866).
Freenome has developed a multi-omic blood test of ctDNA methylation markers for early detection of CRC[41]. The test detects DNA methylation signatures of both tumor-related and immune-related genes. In a recent multi-site study including 122 patients with advanced adenomas and 420 controls, the test had a 41% sensitivity, higher than blood tests for mSEPT9 or stool-based markers, and 90% specificity[42]. The prospective Prevention of Colorectal Cancer Through Multiomics Blood Testing (PREEMPT CRC) study is currently enrolling individuals at “average risk” for CRC to validate the ctDNA-based liquid biopsy test (NCT04369053). Freenome is also investigating blood-based assays of ctDNA to detect changes in transcription factors associated with tumorigenesis[43].
CONCLUSION
The role of liquid biopsy of circulating tumor markers in the early diagnosis of cancer will continue to evolve. Improving technology and decreasing costs could allow liquid biopsy to serve as a highly sensitive and specific tool for aiding in earlier diagnosis of cancer in the near future. Liquid biopsy could one day be used for general population screening and surveillance through preventive health or “wellness checks.” Incorporation of multi-analyte panels into preventive health screening may detect multiple cancer biomarkers simultaneously and move a patient to further workup faster. Screening protocols will need to consider the best ways to incorporate liquid biopsy to benefit patient care, including whether to target specific patient populations or cancer types, how and when results are integrated with other clinical information, and how often testing is needed to benefit patient care[44]. Further studies are needed to not only clinically validate liquid biopsy panels but also understand barriers to their widespread implementation, such as cost, resource use, and feasibility.
DECLARATIONS
Acknowledgments
The authors would like to thank Stacey Tobin, Ph.D., for editorial support in the preparation of this manuscript.
Authors’ contributions
Reviewed the draft manuscript and made substantial revisions: Gawel SH
Conceived of the manuscript topic, wrote the first draft, and revised the manuscript: Davis GJ
Reviewed the draft manuscript and made substantial revision: Jackson L
Reviewed the draft manuscript and made substantial revisions: Jeanblanc N
Approved: Gawel SH, Davis GJ, Jackson L, Jeanblanc N
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by Abbott Laboratories.
Conflicts of interest
All authors are employees of Abbott Laboratories.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2022.
REFERENCES
1. American Cancer Society. Lifetime risk of developing or dying from cancer. 2020; Available from: https://www.cancer.org/cancer/cancer-basics/lifetime-probability-of-developing-or-dying-from-cancer.html. [Last accessed on 29 June 2022].
2. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019;69:363-85.
3. Palmirotta R, Lovero D, Cafforio P, et al. Liquid biopsy of cancer: a multimodal diagnostic tool in clinical oncology. Ther Adv Med Oncol 2018;10:1758835918794630.
4. Morgan TM. Liquid biopsy: Where did it come from, what is it, and where is it going? Investig Clin Urol 2019;60:139-41.
5. Rossi G, Ignatiadis M. Promises and pitfalls of using liquid biopsy for precision medicine. Cancer Res 2019;79:2798-804.
6. Casanova-Salas I, Athie A, Boutros PC, et al. Quantitative and qualitative analysis of blood-based liquid biopsies to inform clinical decision-making in prostate cancer. Eur Urol 2021;79:762-71.
7. Domínguez-Vigil IG, Moreno-Martínez AK, Wang JY, Roehrl MHA, Barrera-Saldaña HA. The dawn of the liquid biopsy in the fight against cancer. Oncotarget 2018;9:2912-22.
8. Li D, Lai W, Fan D, Fang Q. Protein biomarkers in breast cancer-derived extracellular vesicles for use in liquid biopsies. Am J Physiol Cell Physiol 2021;321:C779-97.
9. Werner S, Keller L, Pantel K. Epithelial keratins: Biology and implications as diagnostic markers for liquid biopsies. Mol Aspects Med 2020;72:100817.
10. Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med 2009;361:170-7.
11. Lennon AM, Goggins M. Diagnostic and therapeutic response markers, Pancreatic Cancer. New York, NY: Springer, 2010; pp. 675-701.
12. Locker GY, Hamilton S, Harris J, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol 2006;24:5313-27.
13. Bhardwaj M, Gies A, Werner S, Schrotz-King P, Brenner H. Blood-based protein signatures for early detection of colorectal cancer: a systematic review. Clin Transl Gastroenterol 2017;8:e128.
14. Yang D, Zhang X, Powell CA, et al. Probability of cancer in high-risk patients predicted by the protein-based lung cancer biomarker panel in China: LCBP study. Cancer 2018;124:262-70.
15. Hsu T, Liu T, Gould B, et al. Abstract PO-007: plasma-based detection of pancreatic cancer: A multiomics approach. Cancer Res 2021;81:PO-007.
16. Cai Z, Zhang J, He Y, et al. Liquid biopsy by combining 5-hydroxymethylcytosine signatures of plasma cell-free DNA and protein biomarkers for diagnosis and prognosis of hepatocellular carcinoma. ESMO Open 2021;6:100021.
17. Guo D, Sun J, Wang Y, et al. 956P A multi-analyte liquid biopsy assay integrating cfDNA methylation and protein biomarkers for liver cancer diagnosis. Ann Oncol 2021;32:S828.
18. Sozzi G, Conte D, Mariani L, et al. Analysis of circulating tumor DNA in plasma at diagnosis and during follow-up of lung cancer patients. Cancer Res 2001;61:4675-78.
19. Wroclawski ML, Serpa-Neto A, Fonseca FL, et al. Cell-free plasma DNA as biochemical biomarker for the diagnosis and follow-up of prostate cancer patients. Tumour Biol 2013;34:2921-7.
20. Cohen JD, Li L, Wang Y, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018;359:926-30.
21. Pinsky PF, Prorok PC, Kramer BS. Prostate cancer screening - a perspective on the current state of the evidence. N Engl J Med 2017;376:1285-9.
22. Kleif J, Ferm L, Davis GJ, Christensen IJ, Nielsen HJ. 898 Development of blood-based, cancer-associated biomarkers for early detection of colorectal cancer: significant differences between results from symptomatic and screening subjects. Gastroenterology 2020;158:S-1538.
23. Wen YH, Chang PY, Hsu CM, Wang HY, Chiu CT, Lu JJ. Cancer screening through a multi-analyte serum biomarker panel during health check-up examinations: results from a 12-year experience. Clin Chim Acta 2015;450:273-6.
24. Chung S. False-positive elevations in carcinoembryonic antigen levels at a health screening center. Lab Med Online 2019;9:146.
25. Wang HY, Hsieh CH, Wen CN, Wen YH, Chen CH, Lu JJ. Cancers Screening in an Asymptomatic Population by Using Multiple Tumour Markers. PLoS One 2016;11:e0158285.
26. Wang HY, Chen CH, Shi S, et al. Improving multi-tumor biomarker health check-up tests with machine learning algorithms. Cancers (Basel) 2020;12:1442.
27. National Cancer Institute. Tumor markers in common use. Available from: https://www.cancer.gov/about-cancer/diagnosis-staging/diagnosis/tumor-markers-list [Last accessed on 29 June 2022].
28. Duffy MJ, McDermott EW, Crown J. Blood-based biomarkers in breast cancer: from proteins to circulating tumor cells to circulating tumor DNA. Tumour Biol 2018;40:1010428318776169.
29. Seijo LM, Peled N, Ajona D, et al. Biomarkers in lung cancer screening: achievements, promises, and challenges. J Thorac Oncol 2019;14:343-57.
30. Cargnin S, Canonico PL, Genazzani AA, Terrazzino S. Quantitative analysis of circulating cell-free DNA for correlation with lung cancer survival: a systematic review and meta-analysis. J Thorac Oncol 2017;12:43-53.
31. Qvick A, Stenmark B, Carlsson J, Isaksson J, Karlsson C, Helenius G. Liquid biopsy as an option for predictive testing and prognosis in patients with lung cancer. Mol Med 2021;27:68.
32. Tissot C, Toffart AC, Villar S, et al. Circulating free DNA concentration is an independent prognostic biomarker in lung cancer. Eur Respir J 2015;46:1773-80.
33. González de Aledo-Castillo JM, Casanueva-Eliceiry S, Soler-Perromat A, et al. Cell-free DNA concentration and fragment size fraction correlate with FDG PET/CT-derived parameters in NSCLC patients. Eur J Nucl Med Mol Imaging 2021;48:3631-42.
34. Chaudhuri AA, Chabon JJ, Lovejoy AF, et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov 2017;7:1394-403.
35. Phallen J, Leal A, Woodward BD, et al. Early noninvasive detection of response to targeted therapy in non-small cell lung cancer. Cancer Res 2019;79:1204-13.
36. Sestini S, Boeri M, Marchiano A, et al. Circulating microRNA signature as liquid-biopsy to monitor lung cancer in low-dose computed tomography screening. Oncotarget 2015;6:32868-77.
37. Liu MC. Transforming the landscape of early cancer detection using blood tests-Commentary on current methodologies and future prospects. Br J Cancer 2021;124:1475-7.
38. Duffy MJ, Diamandis EP, Crown J. Circulating tumor DNA (ctDNA) as a pan-cancer screening test: is it finally on the horizon? Clin Chem Lab Med 2021;59:1353-61.
39. Lennon AM, Buchanan AH, Kinde I, et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science 2020;369:eabb9601.
40. Liu MC, Oxnard GR, Klein EA, et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol 2020;31:745-59.
41. Wan N, Weinberg D, Liu TY, et al. Machine learning enables detection of early-stage colorectal cancer by whole-genome sequencing of plasma cell-free DNA. BMC Cancer 2019;19:832.
42. Lin J, Ariazi E, Dzamba M, et al. Evaluation of a sensitive blood test for the detection of colorectal advanced adenomas in a prospective cohort using a multiomics approach. JCO 2021;39:43-43.
43. Ulz P, Perakis S, Zhou Q, et al. Inference of transcription factor binding from cell-free DNA enables tumor subtype prediction and early detection. Nat Commun 2019;10:4666.
44. Putcha G, Gutierrez A, Skates S. Multicancer screening: one size does not fit all. JCO Precis Oncol 2021;5:574-6.
45. Holdenrieder S, Pagliaro L, Morgenstern D, Dayyani F. Clinically meaningful use of blood tumor markers in oncology. Biomed Res Int 2016;2016:9795269.
46. McKeegan EM, Ansell PJ, Davis G, et al. Plasma biomarker signature associated with improved survival in advanced non-small cell lung cancer patients on linifanib. Lung Cancer 2015;90:296-301.
47. Rifai N. Tietz textbook of clinical chemistry and molecular diagnostics, 6th ed. St. Louis, MO: Saunders, 2017; Available from: https://www.us.elsevierhealth.com/tietz-textbook-of-clinical-chemistry-and-molecular-diagnostics-9780323359214.html [Last accessed on 29 June 2022].
48. Lamb YN, Dhillon S. Epi proColon((R)) 2.0 CE: a blood-based screening test for colorectal cancer. Mol Diagn Ther 2017;21:225-32.
49. Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 2014;370:1287-97.
50. Guler GD, Ning Y, Ku CJ, et al. Detection of early stage pancreatic cancer using 5-hydroxymethylcytosine signatures in circulating cell free DNA. Nat Commun 2020;11:5270.
51. Macdonald IK, Parsy-Kowalska CB, Chapman CJ. Autoantibodies: opportunities for early cancer detection. Trends Cancer 2017;3:198-213.
52. Hemken PM, Sokoll LJ, Yang X, et al. Validation of a novel model for the early detection of hepatocellular carcinoma. Clin Proteomics 2019;16:2.
53. Yang JD, Addissie BD, Mara KC, et al. GALAD Score for hepatocellular carcinoma detection in comparison with liver ultrasound and proposal of GALADUS score. Cancer Epidemiol Biomarkers Prev 2019;28:531-8.
Cite This Article
How to Cite
Gawel SH, Jackson L, Jeanblanc N, Davis GJ. Current and future opportunities for liquid biopsy of circulating biomarkers to aid in early cancer detection . J Cancer Metastasis Treat. 2022;8:26. http://dx.doi.org/10.20517/2394-4722.2022.13
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.

























Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.