Immunotherapy in malignant pleural mesothelioma: a long story ended in success
Abstract
Malignant pleural mesothelioma (MPM) is an aggressive and rare disease, mainly due to asbestos exposure, characterized by a poor prognosis. For almost two decades, platinum-based chemotherapy has been the only approved therapeutic regimen for first-line MPM, with an overall survival of 12 months. In the last years, the therapeutic scenario of different tumor types, including MPM, has dramatically changed due to immune checkpoint inhibition. The promising results of this approach have promoted new efforts into clinical research, and many trials investigating novel therapeutic combinations are currently ongoing. The aim of the present review is to provide a comprehensive overview of the most promising immunotherapeutic-based strategies currently under investigation for advanced MPM.
Keywords
INTRODUCTION
Malignant pleural mesothelioma (MPM) is an aggressive disease that affects the pleural membranes lining the lungs, characterized by a poor prognosis. Although it is considered a relatively rare tumor, its global rate increased massively over the last four decades due to asbestos exposure, and its continued slow increase indicates that it has become an epidemic[1]. According to histology, MPM is distinguished into three groups: epithelioid, sarcomatoid, and biphasic type[2,3]. Due to its rapid growth, MPM is generally diagnosed at an advanced stage; consequently, only few patients are suitable for the radical surgical approach (pleuro-pneumonectomy or radical pleural decortication), usually associated with neoadjuvant chemotherapy and adjuvant radiation therapy. Front-line systemic treatment with platinum compounds combined with pemetrexed has been the treatment choice for the majority of MPM patients for almost two decades[4,5]. However, the antitumor efficacy of this regimen is unsatisfactory, with the median overall survival (mOS) of approximately 12 months. Over the last decade, research showed that the angiogenesis process seems to have a key role in MPM progression, which led to the investigation of several antiangiogenetic agents in prospective clinical trials. However, with the exception of the MAPS trial, in which bevacizumab combined with a platinum-based regimen showed a significant improvement in survival compared with chemotherapy alone despite the cardiovascular side effects reported[6], the trials, including the randomized, phase III LUME-Meso-study with nintedanib combined with chemotherapy, failed to demonstrate a survival benefit[7]. Furthermore, the outcome results of second-line regimens approved for relapsed MPM patients were not satisfactory; gemcitabine, vinorelbine, or pemetrexed rechallenge was often utilized in this setting of disease for fit MPM patients, but without a significant improvement in mOS[8].
In the last years, targeting checkpoint inhibitors has dramatically redesigned the therapeutic landscape of different tumor types, and promising results in unresectable MPM subjects, particularly combination regimens, have been recently reported. Consistently, ipilimumab, an anti-cytotoxic T lymphocyte antigen (CTLA)-4 monoclonal antibody (mAb), in combination with nivolumab, an anti-programmed cell death protein (PD)-1 mAb, demonstrated greater efficacy than platinum-based standard regimen in first-line MPM patients, and it has very recently become the new standard of care in several countries[9].
This review critically discusses the recent strategies with immunotherapeutic approaches and those currently under investigation.
METHODS
A comprehensive search strategy of the currently available literature on PubMed, PMC, and NLM databases was performed to identify published studies involving immune checkpoint inhibitors (ICIs) and other immune-therapeutic approaches for the treatment of MPM patients. Furthermore, congress material from the most important oncology conferences held by international associations, including the American Society for Clinical Oncology (ASCO), the International Association for the Study of Lung Cancer (IASLC), the European Society for Medical Oncology (ESMO), and the International Mesothelioma Interest Group, were also evaluated.
Immuno-oncology: immune checkpoint inhibitors
Up to 2020, no treatments succeeded the platinum-pemetrexed combination regimen in association with or without bevacizumab as a first-line option for unresectable MPM[5,6,8].
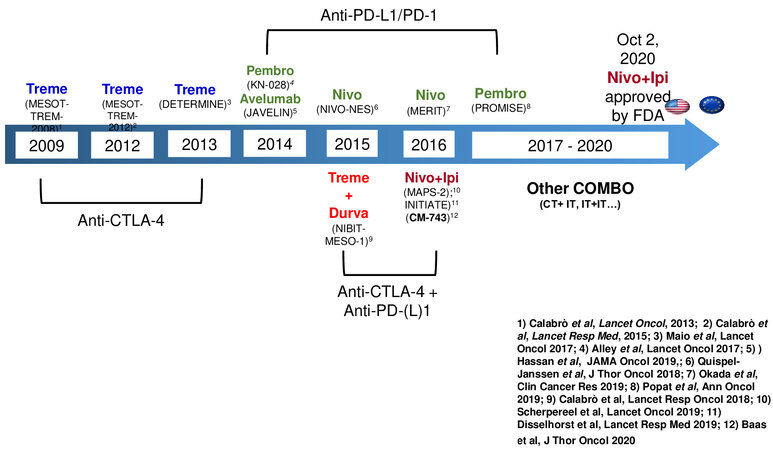
Indeed, new effective therapeutic agents have been frustratingly slow to develop. This dramatic scenario has recently changed with the phase III CheckMate 743 trial[10], which showed a statistically significant improvement in survival with nivolumab plus ipilimumab compared to chemotherapy, thereby licensing this new regimen as the first choice for advanced MPM subjects in most countries. This approval followed a long course of clinical studies with CTLA-4/PD-1/PD-ligand(L)-1 blocking agents used alone or in combination showing signs of antitumor activity [Figure 1], mostly conducted in pretreated MPM patients, a setting in which therapeutic options are very limited[11,12].
Targeting CTLA-4
The phase II MESOT-TREM 2008 trial was the first study that opened the path towards this novel therapeutic approach. In this study, 29 pretreated MPM and peritoneal malignant mesothelioma patients underwent anti-CTLA-4 mAb tremelimumab therapy at the dose of 15 mg/kg every 90 days until disease progression or unacceptable toxicity. Although the primary endpoint was not reached due to the low ORR (6.9%), preliminary signs of antitumor activity were noted, in particular in terms of mOS which was 10.7 months[13]. Therefore, a second study, MESOT-TREM 2012, started. In this phase II trial, based on the pharmacokinetic analysis generated in previous studies[14], tremelimumab was given at the intensified dose of 10 mg/kg every four weeks for six cycles, followed by maintenance every 12 weeks. Opposite to MESOT-TREM-2008, in this study, the primary endpoint was reached with an immune-related ORR of 13.8%[15]. Moreover, the study showed a good safety profile, with grade 3-4 toxicity observed in only 7% of treated patients[15]. In light of these promising results, the phase IIb, placebo-controlled, double-blind DETERMINE study started. Overall, 568 patients affected by pretreated MPM or peritoneal malignant mesothelioma were randomized to receive tremelimumab, given at the same intensified dose utilized in the MESOT-TREM-2012 study, or placebo. Unfortunately, the primary endpoint was not reached: tremelimumab failed to demonstrate an improvement in OS, compared to placebo (7.7 and 7.3 months, respectively; HR = 0.92;
Targeting PD1/PDL1 axis
The development of a second generation of immunomodulating mAb directed against the PD-1/PD-L1 axis aroused keen interest to explore in MPM patients because of their more promising profile in efficacy and safety compared to that of anti-CTLA-4 mAb in different tumor types. Thus, various phase I/II trials were conducted in MPM patients[11,12,17-21]. Among the most representative studies, the phase Ib Keynote 028 study (NCT02054806) was conducted on 25 MPM patients treated with pembrolizumab. The results show a response rate of 20% and a mOS of 18 months[20].
In the MERIT II study, a flat dose of nivolumab administration (240 mg every two weeks) was evaluated in 34 Japanese pretreated MPM patients. The mOS was 17.3 months, three-year survival was 23.5%, mPFS was 6.1 months, and ORR was 29%, regardless of the histotype[21]. Because of these results, nivolumab received approval as second-line therapy for MPM patients in Japan[21].
Following these positive results, two phase III studies, PROMISE-Meso and CONFIRM, started[22,23]. In the first one, 144 unresectable, pretreated MPM patients were randomized to receive chemotherapy (gemcitabine or vinorelbine) or pembrolizumab. Crossover to pembrolizumab was allowed. After a median follow-up of 11.8 months, no differences were seen in mOS and mPFS between pembrolizumab and chemotherapy (mOS, 10.7 vs. 12.4 months; HR = 1.12; 95%CI: 0.74-1.69; P = 0.59; mPFS, 2.5 vs. 3.4 months; HR = 1.06; 95%CI: 0.73-1.53; P = 0.76)[21]. However, in the group treated with pembrolizumab, an increase in response rate was recorded (22% vs. 6% treated with chemotherapy, P = 0.004). The PD-L1 expression was not predictive of better survival with pembrolizumab[22].
In the CONFIRM trial, 332 second- or third-line MPM patients were randomized to receive nivolumab or placebo. In this trial, cross-over was not permitted. Median OS was slightly higher with nivolumab than in the placebo group (9.2 vs. 6.6 months; HR = 0.72; 95%CI: 0.55-0.94; P = 0.018)[23]. Opposite to CheckMate 743, an improvement in OS was seen in patients with epithelioid histology (9.4 vs. 6.6; HR = 0.71; 95%CI: 0.53-0.95; P = 0.021) but not in those with non-epithelioid histology (5.9 vs. 6.7 months; HR = 0.79; 95%CI: 0.35-1.79; P = 0.572)[8,22]. The grade 3 and 4 adverse event rates were 13.1% in the nivolumab arm and 2.7% in the placebo arm[23].
Although the results generated from these studies demonstrate an overall antitumor efficacy of CTLA-4 or PD-1/PD-L1 inhibition, the latter seems to be limited to a subgroup of subjects, probably because the immune-modulating effect of these agents may not be enough to overcome the strong immunosuppressive microenvironment of MPM if used as monotherapy.
Co-targeting of CTLA-4 and PD1/PDL1 axis
In the last few years, great efforts have been made on combination regimens targeting ICIs to enhance the efficacy of immunotherapeutic agents and overcome primary resistance to treatment observed in a large proportion of cancer patients. Several mechanisms of resistance have been thus far identified[24], and strategies to overcome them represent an area of strong investigation.
Along this line, co-targeting CTLA-4 and PD-1/PD-L1 axis represents an optimal combination regimen in view of a complementary mechanism of action of these molecules. Indeed, CTLA-4 and PD-1/PD-L1 molecules act in two different phases of T-cell activation; therefore, they are non-redundant and cooperative pathways. The antitumor efficacy of CTLA-4 and PD-1/PD-L1 blockade has been largely investigated and, recently, obtained approval for several cancer types, including microsatellite instability (MSI)-positive colorectal cancer (CRC), renal cell carcinoma, non-small cell lung cancer (NSCLC) (in combination with chemotherapy), and gastrointestinal cancer[25,26].
NIBIT-MESO-1 was the first study that investigated the potential efficacy of ICI combination regimens in mesothelioma patients. In this phase II study, 40 MPM or peritoneal mesothelioma patients received, in first or second line, tremelimumab at 1 mg/kg in combination with durvalumab at 20 mg/kg every four weeks for four cycles (induction phase), for non- progressor patients, followed by maintenance with only durvalumab, at the same dose, every 4 weeks for a maximum of nine cycles. Noteworthy, patients who experienced disease progression during the maintenance or follow-up phase were allowed to restart the combination treatment. The study reached its primary endpoint with an immune-related objective response of 28%; the median duration of response was 16.1 months, DCR was 65%, and mOS was 16.5 months[27]. The three- and four-year OS rates were 20% and 15%, respectively[28]. Among the 40 patients enrolled in the NIBIT-MESO-1 study, 17 met the criteria for retreatment. Interestingly, 41% of retreated patients achieved a DCR; moreover, no grade 3-4 immune-related adverse events were observed[28]. Seeking to identify predictive biomarkers for patient selection to retreatment, an assessment of tumor mutational burden (TMB) was performed, and the results show a significantly (P = 0.02) higher mOS for retreated patients with a TMB over the median (41.3 months) compared with those who had a TMB level under the median one (17.4 months)[28].
Following the NIBIT-MESO-1 study, two additional combination trials have been conducted in MPM patients. In the IFCT-1501 MAPS-2 study, 54 patients received nivolumab plus ipilimumab. Overall,
Most representative clinical trials on ICIs alone or in combination
| Regimen | Trial design | Patients (N°) | Results | Refs. |
| IPI-NIVO vs. Chemotherapy | Phase III Randomized 1st line | 605 | mOS 18.1 vs. 14.1 months; HR, 0.74; P = 0.002 | [10] |
| IPI-NIVO vs. NIVO | Phase II Randomized 2nd and further line | 125 | mOS 15.9 vs. 11.9 months | [29] |
| IPI-NIVO | Phase II Not-Randomized 2nd and further line | 34 | SD 38%; mOS: not reached | [30] |
| Durvalumab plus chemotherapy | Phase II trial Single arm 1st line | 55 | Median OS: 21.0 months | [39] |
| Durvalumab plus chemotherapy | Phase II trial Single arm 1st line | 55 | 6-month PFS: 57% | [40] |
| Durvalumab plus tremelimumab | Phase II trial Single arm 1st line or pretreated | 40 | Immune-related ORR: 28% DCR 65%; mOS: 16.5 months | [28] |
| NIVO | Phase II trial Single arm 2nd and further line | 34 | ORR: 29% mOS: 17.3 months | [12] |
| NIVO vs. Placebo | Phase II Randomized 2nd and further line | 332 | mOS 9.2 months vs. 6.6 months; HR: 0.72 1-year OS: 43.4% vs. 30.1% | [23] |
| Pembrolizumab vs. Institutional choice | Phase III Randomized 2nd and further line | 144 | mOS 10.7 months vs. 12.4 months; HR: 1.12; P = 0.59 ORR: 22% vs. 6% ; P = 0.004 | [22] |
| Pembrolizumab | Phase Ib Single arm 2nd and further line | 25 | ORR 20%; mOS: 18 months | [20] |
| Tremelimumab | Phase II trial Single arm 2nd and further Line | 29 | ORR: 6.9% mOS: 10.7 months | [15] |
| Tremelimumab | Phase II trial Single arm 2nd and further line | 29 | Immune-related ORR: 13.8% (4 patients) | [13] |
| Tremelimumab vs. Placebo | Phase II Randomized 2nd and further line | 571 | mOS 7.7 months vs. 7.3 months; HR: 0.92; P = 0.41 | [16] |
The results generated from the NIBIT-MESO-1, MAPS-2, and INITIATE studies strongly contributed to the activation of the randomized, phase III CheckMate 743 trial[10]. In this study, 605 not pretreated MPM patients were randomized (1:1) to receive nivolumab, at the dosage of 3 mg/kg every two weeks, in combination with ipilimumab, at the dosage of 1 mg/kg every six weeks, for up to two years or the standard first-line treatment with platinum compound and pemetrexed combination for a maximum of six cycles. In the primary analysis, a statistically significant improvement in OS (the primary aim of the trial) was seen in the immunotherapy arm compared with the chemotherapy one, with a mOS of 18.1 vs. 14.1 months
Although no significant differences were seen in median progression-free survival (PFS) and objective response rates (ORR) between the two arms, the median duration of response was significantly longer with combination immunotherapy (11.0 vs. 6.7 months). Grade 3 and 4 treatment-related adverse events were similar in the ICI (30%) and chemotherapy groups (32%). However, the incidence and severity of the adverse events led to a greater rate of discontinuation in the ICI group (15%) than in the chemotherapy group (7%), and three treatment-related deaths were reported in the ICI group compared with only one in the chemotherapy arm[10]. Concerning these latter aspects, the lesser experience of many oncologists in immune-related toxicity management might have contributed to this difference. In the ICI arm, the most frequent toxicities observed were dermatological, gastrointestinal, endocrine, hepatic, and pulmonary. Noteworthy, approximately 35% of patients who discontinued treatment with nivolumab and ipilimumab due to the onset of side effects had a persistent objective response and an improvement in mOS (25.4 months) compared to those observed in all patients treated with ICIs (18.1 months)[10]. Furthermore, nivolumab and ipilimumab combination improved disease-related symptoms and maintained QoL in patients with advanced MPM[31]. A three-year survival update showed a persistent benefit in survival for patients treated with ICI compared to those treated with chemotherapy (23% vs. 15%)[32]. The benefit was seen across subgroups including histology. Exploratory biomarker analyses demonstrated that a high score on a four-gene inflammatory signature (CD8A, STAT1, LAG3, and CD274) is correlated with better survival in the experimental arm than in the standard one[32]. The CheckMate 743 results led to the Food and Drug Administration (FDA) approval of nivolumab and ipilimumab combination in MPM patients in October 2020, followed by the European Medicines Agency (EMA) approval in June 2021[33].
Is chemotherapy combined with ICI the next step?
Great efforts are currently addressing novel combination regimens to enhance the efficacy of immunotherapy and overcome primary resistance to treatment, largely observed in most cancer patients[34-38]. Along this line, chemotherapy could be an interesting partner for a combination regimen with immunotherapy, in view of its immunomodulatory properties. Currently, different trials investigating the efficacy of platinum-pemetrexed combined with ICIs are ongoing.
In the phase II PrE505 study, first-line MPM patients received treatment with platinum-pemetrexed in combination with the anti-PD-L1 durvalumab[39]. Updated results show a remarkable mOS of 20.4 months (95%CI: 13.0-28.5) with a one-year OS rate of 70.4%[39]. Furthermore, the study provided an interesting ORR of 56.4% (95%CI: 42.3-69.7%). In the phase II Dream study, patients were treated with a platinum-based regimen plus pemetrexed in combination with durvalumab used at the fixed dose of 1125 mg for six cycles, followed by durvalumab maintenance for up to one year. The study showed a six-month PFS rate of 57%[40]. Based on these promising results, the phase III Dream3r study is ongoing[41].
Two additional studies are currently ongoing: In the randomized, front-line phase III IND227 trial (NCT02784171), MPM patients receive platinum-based chemotherapy plus the anti-PD-1 pembrolizumab or chemotherapy alone. The study has completed the accrual, and the results are awaited. The actively recruiting phase III BEAT-Meso study s exploring the efficacy of chemotherapy plus bevacizumab and atezolizumab vs. only chemotherapy in first-line MPM patients (NCT03762018).
Results from the phase III studies are urgently awaited, and if positive, they could enrich the therapeutic approaches in this setting of disease.
Immune-oncology: not only ICI
Mesothelin (MSLN) is a membrane protein predominantly expressed in both normal and malignant mesothelial cells, above all in the epithelioid histological subtype. For these reasons, it appears to be an optimal candidate for targeted therapies[42]. The preliminary antitumor effect observed in preclinical studies led to the design of a phase I trial using T cells expressing a second-generation murine anti-mesothelin chimeric antigen receptor (CAR)[43,44]. No clinical response or “on target” toxicities were recorded, but interestingly an immunogenicity reaction to the murine SS1 scFv used in the CAR construct was noted[45]. Given these premises, a second phase I trial (NCT02159716) was conducted. Fifteen patients affected by different tumor types including mesothelioma received a lentiviral transduction vector expressing a second-generation murine-based anti-mesothelin CAR[46]. Cyclophosphamide was used as pretreatment to enhance CAR-T expansion. No complete responses (CR) or PR were reported, but a SD was found in 11/15 patients[46]. At the University of Pennsylvania, another trial is ongoing using both intravenous and intrapleural administration of a fully human anti-mesothelin CAR in combination with cyclophosphamide (NCT03054298). In a recently published phase I/II trial, mesothelin-targeting CAR-T cells were safely administered with an intrapleural injection to 31 patients, with no procedure-related adverse events greater than grade 1[47].
In another phase I/II trial, 25 pretreated MPM patients received an intrapleural administration of a MSLN targeting CAR T cell therapy either alone or followed by pembrolizumab[48]. Pembrolizumab was administered after CAR-T cell therapy in 18 patients. Promising results were observed, with an mOS of 23.9 months and a one-year OS of 83%[48]. Together with MSLN, fibroblast activation protein (FAP) and Wilms tumor 1 (WT1) also represent promising targets for CAR-T cell therapies in MPM. FAP is highly expressed in the tumor microenvironment, particularly in cancer-associated stromal cells[49]. In a phase I trial, the anti-FAP CD8+ CAR-T cell intrapleural administration tested on three patients was safe (NCT01722149). No serious adverse events were reported[50,51]. WT1 is a protein expressed in both epithelioid and non-epithelioid MPM. An ongoing phase I/II trial using anti-WT1 TCR T cells in MM patients expressing WT1 and human leukocyte antigen (HLA)-A*0201 is ongoing. To increase their efficacy, central memory and naïve CD8+ T cells have been selected for TCR transduction (NCT02408016). Other promising targets tested in preclinical studies include MET[52], Pan-ErbB T4[53], 5 T4[54], chondroitin sulfate proteoglycan 4 (CSPG4), and angiogenesis[55,56]. Therapeutic cancer vaccines represent another attractive therapeutic approach that aims to stimulate an antitumor immune response with the administration of engineered dendritic cells (DCs), genetic material, or peptides[57]. A long-lasting experience in DC vaccine has permitted the development of this approach in MPM. Based on promising preclinical results generated in mouse models[58], a phase I study with DC vaccine in MPM patients was conducted (NCT00280982). In the study, three patients (30%) obtained a partial response, and overall, the treatment showed a good safety profile[59]. In another phase I clinical trial, the autologous DC vaccine was combined with low-dose cyclophosphamide in 10 pretreated MPM patients (NCT01241682). The combination regimen showed a good safety profile and an antitumor effect in 7/10 patients, obtaining a survival of ≥ 2 years. After 50 and 66 months, 2 of 10 patients were alive[60]. Due to these impressive, although preliminary results, a phase II/III trial (NCT03610360) is ongoing. This trial is evaluating DCs loaded with allogeneic tumor cell lysates as maintenance therapy after first-line chemotherapy (DENIM trial), having OS as the primary endpoint[61].
An additional attractive study is the phase Ib MESOVAX (NCT03546426), i.e., investigating the efficacy of an autologous DC vaccine in combination with pembrolizumab in pretreated MPM or peritoneal mesothelioma patients. The study is currently recruiting.
Genetic vaccines, including DNA, RNA, and viral-based vaccines, aim to improve the antigen-specific antitumor immune response[62-67].
Several types of oncolytic viruses have been tested in various preclinical and clinical trials for the treatment of MPM[66,67]. Currently, a phase I clinical study is investigating the efficacy and safety of the intrapleural administration of an oncolytic measles virus (MV-NIS virus) in 15 MPM patients (NCT01503177).
WT1 is also considered as a potential target, not only for the development of CAR therapy but also for peptide vaccines in MPM patients[68]. In a randomized, phase II clinical trial (NCT01265433), pretreated MPM patients were randomized to receive analog WT1 peptide vaccine galinpepimut-S or placebo. The results show an increase in mPFS and mOS of 36% and 25%, respectively, in patients treated with the vaccine compared to the placebo group[69].
Based on these promising results, there is currently an ongoing phase I study (NCT04040231) investigating the safety of galinpepimut-S alone or in combination with nivolumab in pretreated MPM patients.
Additionally, in a phase I clinical trial (NCT01675765), the safety and efficacy of the sequential administration of CRS-207, an attenuated vaccine of Listeria monocytogenes genetically modified and engineered to stimulate an immune response against mesothelin (with or without cyclophosphamide) and chemotherapy, was investigated in MPM patients. The results demonstrate that CRS-207 administration is safe and the subsequent administration of cisplatin and pemetrexed is well tolerated. In particular, no patients developed listeriosis. Moreover, a PR was reported in 54% and a SD in 29% of patients enrolled; mPFS and mOS were 7.5 and 14.7 months, respectively[70].
CONCLUSION
For decades, no progress whatsoever has been made in MPM, and many trials have failed to demonstrate the efficacy of new treatments[71]. A better knowledge of tumor biology and its interactions with immune cells and tumor microenvironment has recently led to a therapeutic paradigm shift in MPM, with the entrance to the clinic of the first chemotherapy-free regimen based on the association of nivolumab plus ipilimumab[10,72]. Based on this, immunotherapy is no longer regarded as “Cinderella” and has became the “Princess” therapy in MPM, giving new lifeblood to the clinical research on this tumor.
Certainly, much has to be gained to overcome the immune resistance to ICI; along this line, new ICI-based regimens or other agents such as anti-angiogenic compounds or cancer vaccines are currently under active investigation and will hopefully play a leading role in the treatment of mesothelioma[73,74].
Finally, the “one size fits all” approach is not recommended for patients with MPM; therefore, the identification of validated biomarkers is mandatory to select patients for the best treatment based on the characteristics of their tumor and the associated microenvironment.
DECLARATIONS
AcknowledgmentsThis work was partly supported thanks to the contribution of Ricerca Corrente by the Italian Ministry of Health within the research line L1P.
Authors’ contributionsWrote and revised the text: Bongiovanni A
Revised the text: Frassoldati A
Wrote and revised the text: Calabrò L
All authors provided comments on the initial version and approved the final draft of the manuscript.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestBongiovanni A. has served as advisor for Novartis/AAA and he has received funds from Amgen for a translational study. Frassoldati A. declare no conflict of interest; Calabrò L. has served as consultant or advisor to Bristol-Myers Squibb, Merck Sharp and Dohme, and received compensated educational activities from Bristol Myers Squibb, Astrazeneca, Sanofi.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Milano MT, Zhang H. Malignant pleural mesothelioma: a population-based study of survival. J Thorac Oncol 2010;5:1841-8.
2. Beasley MB, Galateau-Salle F, Dacic S. Pleural mesothelioma classification update. Virchows Arch 2021;478:59-72.
3. Husain AN, Colby TV, Ordóñez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the international mesothelioma interest group. Arch Pathol Lab Med 2018;142:89-108.
4. Viscardi G, Di Liello R, Morgillo F. How I treat malignant pleural mesothelioma. ESMO Open 2020;4:e000669.
5. Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44.
6. Zalcman G, Mazieres J, Margery J, et al. French cooperative thoracic intergroup (IFCT), bevacizumab for newly diagnosed pleural mesothelioma in the mesothelioma avastin cisplatin pemetrexed study (MAPS): a randomised, controlled, open- label, phase 3 trial. Lancet 2016;387:1405-14.
7. Scagliotti GV, Gaafar R, Nowak AK, et al. Nintedanib in combination with pemetrexed and cisplatin for chemotherapy-naive patients with advanced malignant pleural mesothelioma (LUME-Meso): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet Respir Med 2019;7:569-80.
8. Davis AP, Kao SC, Clarke SJ, Boyer M, Pavlakis N. Emerging biological therapies for the treatment of malignant pleural mesothelioma. Expert Opin Emerg Drugs 2021;26:179-92.
9. Ge Z, Peppelenbosch MP, Sprengers D, Kwekkeboom J. TIGIT, the next step towards successful combination immune checkpoint therapy in cancer. Front Immunol 2021;12:699895.
10. Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021;397:375-86.
11. Yap TA, Nakagawa K, Fujimoto N, et al. Efficacy and safety of pembrolizumab in patients with advanced mesothelioma in the open-label, single-arm, phase 2 KEYNOTE-158 study. Lancet Respir Med 2021;2021:S2213260020305154.
12. Okada M, Kijima T, Aoe K, et al. Clinical efficacy and safety of nivolumab: results of a multicenter, open-label, single-arm, japanese phase ii study in malignant pleural mesothelioma (MERIT). Clin Cancer Res 2019;25:5485-92.
13. Calabrò L, Morra A, Fonsatti E, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol 2013;14:1104-11.
14. Calabrò L, Ceresoli GL, di Pietro A, et al. CTLA4 blockade in mesothelioma: finally a competing strategy over cytotoxic/target therapy? Cancer Immunol Immunother 2015;64:105-12.
15. Calabrò L, Morra A, Fonsatti E, et al. Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, single-arm, phase 2 study. Lancet Respir Med 2015;3:301-9.
16. Maio M, Scherpereel A, Calabrò L, et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol 2017;18:1261-73.
17. Calabrò L, Ceresoli GL, D’Incecco A, Scherperee A, Aerts J, Maio M. Immune checkpoint therapy of mesothelioma: pre-clinical bases and clinical evidences. Cytokine Growth Factor Rev 2017;36:25-31.
18. Quispel-Janssen J, van der Noort V, de Vries JF, et al. Programmed death 1 blockade with nivolumab in patients with recurrent malignant pleural mesothelioma. J Thorac Oncol 2018;13:1569-76.
19. Hassan R, Thomas A, Nemunaitis JJ, et al. Efficacy and safety of avelumab treatment in patients with advanced unresectable mesothelioma: phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol 2019;5:351-7.
20. Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 2017;18:623-30.
21. Fujimoto N, Okada M, Kijima T, et al. Clinical efficacy and safety of nivolumab in Japanese patients with malignant pleural mesothelioma: 3-year results of the MERIT study. JTO Clin Res Rep 2021;2:100135.
22. Popat S, Curioni-Fontecedro A, Dafni U, et al. A multicentre randomised phase III trial comparing pembrolizumab versus single-agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: the European Thoracic Oncology Platform (ETOP 9-15) PROMISE-meso trial. Ann Oncol 2020;31:1734-45.
23. Fennell DA, Ewings S, Ottensmeier C, et al. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol 2021;22:1530-40.
24. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016;16:275-87.
25. Chen J, Li S, Yao Q, et al. The efficacy and safety of combined immune checkpoint inhibitors (nivolumab plus ipilimumab): a systematic review and meta-analysis. World J Surg Oncol 2020;18:150.
26. Arru C, De Miglio MR, Cossu A, et al. Durvalumab plus tremelimumab in solid tumors: a systematic review. Adv Ther 2021;38:3674-93.
27. Calabrò L, Morra A, Giannarelli D, et al. Tremelimumab combined with durvalumab in patients with mesothelioma (NIBIT-MESO-1): an open-label, non-randomised, phase 2 study. Lancet Respir Med 2018;6:451-60.
28. Calabrò L, Rossi G, Morra A, et al. Tremelimumab plus durvalumab retreatment and 4-year outcomes in patients with mesothelioma: a follow-up of the open label, non-randomised, phase 2 NIBIT-MESO-1 study. Lancet Respir Med 2021;9:969-76.
29. Scherpereel A, Mazieres J, Greillier L, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol 2019;20:239-53.
30. Disselhorst MJ, Quispel-janssen J, Lalezari F, et al. Ipilimumab and nivolumab in the treatment of recurrent malignant pleural mesothelioma (INITIATE): results of a prospective, single-arm, phase 2 trial. Lancet Respir Med 2019;7:260-70.
31. Scherpereel A, Antonia S, Bautista Y, et al. First-line nivolumab plus ipilimumab versus chemotherapy for the treatment of unresectable malignant pleural mesothelioma: patient-reported outcomes in CheckMate 743. Lung Cancer 2022;167:8-16.
32. Peters S, Scherpereel A, Cornelissen R, et al. First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann Oncol 2022;33:488-99.
33. Wright K. FDA approves nivolumab plus ipilimumab for previously untreated unresectable malignant pleural mesothelioma. Oncology 2020;34:502-3.
34. Kaur J, Elms J, Munn AL, Good D, Wei MQ. Immunotherapy for non-small cell lung cancer (NSCLC), as a stand-alone and in combination therapy. Crit Rev Oncol Hematol 2021;164:103417.
35. Albittar AA, Alhalabi O, Glitza Oliva IC. Immunotherapy for melanoma. Adv Exp Med Biol 2020;1244:51-68.
36. Esposito G, Palumbo G, Carillio G, et al. Immunotherapy in small cell lung cancer. Cancers 2020;12:2522.
37. Ganesh K, Stadler ZK, Cercek A, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol 2019;16:361-75.
39. Forde PM, Anagnostou V, Sun Z, et al. Durvalumab with platinum-pemetrexed for unresectable pleural mesothelioma: survival, genomic and immunologic analyses from the phase 2 PrE0505 trial. Nat Med 2021;27:1910-20.
40. Nowak AK, Lesterhuis WJ, Kok P, et al. Durvalumab with first-line chemotherapy in previously untreated malignant pleural mesothelioma (DREAM): a multicentre, single-arm, phase 2 trial with a safety run-in. Lancet Oncol 2020;21:1213-23.
41. Kok PS, Forde PM, Hughes B, et al. Protocol of DREAM3R: DuRvalumab with chEmotherapy as first-line treAtment in advanced pleural Mesothelioma-a phase 3 randomised trial. BMJ Open 2022;12:e057663.
42. Ordóñez NG. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod Pathol 2003;16:192-7.
43. Beatty GL, Haas AR, Maus MV, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce antitumor activity in solid malignancies. Cancer Immunol Res 2014;;2:112-20.
44. Wang X, Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics 2016;3:16015.
45. Maus MV, Haas AR, Beatty GL, et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res 2013;1:26-31.
46. Haas AR, Tanyi JL, O’Hara MH, et al. Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol Ther 2019;27:1919-29.
47. Ghosn M, Cheema W, Zhu A, et al. Image-guided interventional radiological delivery of chimeric antigen receptor (CAR) T cells for pleural malignancies in a phase I/II clinical trial. Lung Cancer 2022;165:1-9.
48. Adusumilli PS, Zauderer MG, Riviere I, et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discov 2021;11:2768.
49. Schuberth PC, Hagedorn C, Jensen SM, et al. Treatment of malignant pleural mesothelioma by fibroblast activation protein-specific re-directed T cells. J Transl Med 2013;11:187.
50. Petrausch U, Schuberth PC, Hagedorn C, et al. Re-directed T cells for the treatment of fibroblast activation protein (FAP)-positive malignant pleural mesothelioma (FAPME-1). BMC Cancer 2012;12:615.
51. Curioni A, Britschgi C, Hiltbrunner S, et al. A phase I clinical trial of malignant pleural mesothelioma treated with locally delivered autologous anti-FAP-targeted CAR T-cells. Ann Oncol 2019;30:v501.
52. Thayaparan T, Petrovic RM, Achkova DY, et al. CAR T-cell immunotherapy of MET-expressing malignant mesothelioma. OncoImmunology 2017;6:e1363137.
53. Klampatsa A, Achkova DY, Davies DM, et al. Intracavitary “T4 immunotherapy” of malignant mesothelioma using pan-ErbB re-targeted CAR T-cells. Cancer Lett 2017;393:52-9.
54. Al-Taei S, Salimu J, Lester JF, et al. Overexpression and potential targeting of the oncofoetal antigen 5T4 in malignant pleural mesothelioma. Lung Cancer 2012;77:312-8.
55. Beard RE, Zheng Z, Lagisetty KH, et al. Multiple chimeric antigen receptors successfully target chondroitin sulfate proteoglycan 4 in several different cancer histologies and cancer stem cells. J Immunother Cancer 2014;2:25.
56. Akbari P, Huijbers EJM, Themeli M, Griffioen AW, van Beijnum JR. The tumor vasculature an attractive CAR T cell target in solid tumors. Angiogenesis 2019;22:473-5.
57. Song Q, Zhang CD, Wu XH. Therapeutic cancer vaccines: from initial findings to prospects. Immunol Lett 2018;196:11-21.
58. Hegmans JP, Hemmes A, Aerts JG, Hoogsteden HC, Lambrecht BN. Immunotherapy of murine malignant mesothelioma using tumor lysate-pulsed dendritic cells. Am J Respir Crit Care Med 2005;171:1168-77.
59. Hegmans JP, Veltman JD, Lambers ME, et al. Consolidative dendritic cell-based immunotherapy elicits cytotoxicity against malignant mesothelioma. Am J Respir Crit Care Med 2010;181:1383-90.
60. Cornelissen R, Hegmans JP, Maat AP, et al. Extended tumor control after dendritic cell vaccination with low-dose cyclophosphamide as adjuvant treatment in patients with malignant pleural mesothelioma. Am J Respir Crit Care Med 2016;193:1023-31.
61. Belderbos RA, Baas P, Berardi R, et al. A multicenter, randomized, phase II/III study of dendritic cells loaded with allogeneic tumor cell lysate (MesoPher) in subjects with mesothelioma as maintenance therapy after chemotherapy: DENdritic cell immunotherapy for mesothelioma (DENIM) trial. Transl Lung Cancer Res 2019;8:280-5.
62. Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev 2011;239:62-84.
63. Yang B, Jeang J, Yang A, Wu TC, Hung CF. DNA vaccine for cancer immunotherapy. Hum Vaccin Immunother 2014;10:3153-64.
64. Kreiter S, Diken M, Selmi A, Türeci Ö, Sahin U. Tumor vaccination using messenger RNA: prospects of a future therapy. Curr Opin Immunol 2011;23:399-406.
65. McNamara MA, Nair SK, Holl EK. RNA-based vaccines in cancer immunotherapy. J Immunol Res 2015;2015:794528.
67. Msaouel P, Opyrchal M, Dispenzieri A, et al. Clinical trials with oncolytic measles virus: current status and future prospects. Curr Cancer Drug Targets 2018;18:177-87.
68. Eguchi T, Kadota K, Mayor M, et al. Cancer antigen profiling for malignant pleural mesothelioma immunotherapy: expression and coexpression of mesothelin, cancer antigen 125, and Wilms tumor 1. Oncotarget 2017;8:77872-82.
69. Zauderer MG, Tsao AS, Dao T, et al. A randomized phase II trial of adjuvant galinpepimut-S, WT-1 analogue peptide vaccine, after multimodality therapy for patients with malignant pleural mesothelioma. Clin Cancer Res 2017;23:7483-9.
70. Hassan R, Alley E, Kindler H, et al. Clinical response of live-attenuated, Listeria monocytogenes expressing mesothelin (CRS-207) with chemotherapy in patients with malignant pleural mesothelioma. Clin Cancer Res 2019;25:5787-98.
71. Rossini M, Rizzo P, Bononi I, et al. New perspectives on diagnosis and therapy of malignant pleural mesothelioma. Front Oncol 2018;8:91.
72. Harber J, Kamata T, Pritchard C, Fennell D. Matter of TIME: the tumor-immune microenvironment of mesothelioma and implications for checkpoint blockade efficacy. J Immunother Cancer 2021;9:e003032.
73. Rijavec E, Biello F, Barletta G, Dellepiane C, Genova C. Novel approaches for the treatment of unresectable malignant pleural mesothelioma: a focus on immunotherapy and target therapy (Review). Mol Clin Oncol 2022;16:89.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Bongiovanni A, Frassoldati A, Calabrò L. Immunotherapy in malignant pleural mesothelioma: a long story ended in success. J Cancer Metastasis Treat 2022;8:44. http://dx.doi.org/10.20517/2394-4722.2022.78
AMA Style
Bongiovanni A, Frassoldati A, Calabrò L. Immunotherapy in malignant pleural mesothelioma: a long story ended in success. Journal of Cancer Metastasis and Treatment. 2022; 8: 44. http://dx.doi.org/10.20517/2394-4722.2022.78
Chicago/Turabian Style
Bongiovanni, Alberto, Antonio Frassoldati, Luana Calabrò. 2022. "Immunotherapy in malignant pleural mesothelioma: a long story ended in success" Journal of Cancer Metastasis and Treatment. 8: 44. http://dx.doi.org/10.20517/2394-4722.2022.78
ACS Style
Bongiovanni, A.; Frassoldati A.; Calabrò L. Immunotherapy in malignant pleural mesothelioma: a long story ended in success. J. Cancer. Metastasis. Treat. 2022, 8, 44. http://dx.doi.org/10.20517/2394-4722.2022.78
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 12 clicks
Cite This Article 12 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.