Assessment of response to liver directed radiation-based therapies: Current guidelines, challenges, and future directions
Abstract
Radiation-based local-regional therapies for hepatocellular carcinoma (HCC) have gained wide acceptance due to promising rates of tumor response, survival, and safety profiles. After treatment, it is important to assess tumor response to determine further management, patient prognosis, and endpoint outcomes for clinical trials. To standardize imaging interpretation and reporting of HCC response to local-regional treatment, a few imaging-based response assessment systems were developed. Two of them have emerged as the most used: the Liver Imaging Reporting and Data System (LI-RADS) Treatment Response Algorithm (LR-TRA) and the modified Response Evaluation Criteria in Solid Tumors (mRECIST). While these systems have been validated for the assessment of response to ablative locoregional therapies, assessment of response to radiation-based therapies can be challenged by persistent or evolving imaging features and is still an area of active research. Following the advances in technology and a better understanding of tumor biology that allowed for the increased application of radiation-based local-regional therapies for the treatment of HCC, research is still needed to address the limitations of current imaging criteria for assessing tumor response to these novel techniques. In this review, we describe radiation-based liver-directed treatment options, examine imaging criteria for assessing treatment response, discuss practical limitations and gaps in knowledge when applying these response criteria, and address future directions that may help to improve accuracy and outcomes when assessing response to radiation-based HCC treatment.
Keywords
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common form of primary liver malignancy and the third most common cause of cancer-related death in 2020[1]. The most important risk factor for the development of HCC is underlying chronic liver disease, which may be related to alcohol consumption, hepatitis B or C infection, or nonalcoholic steatohepatitis (NASH)[2]. In patients at high risk for HCC development, imaging surveillance is recommended[3], most often with biannual liver ultrasound (US) exams. A positive surveillance US exam is followed by contrast-enhanced multiphase computer tomography (CT) or magnetic resonance imaging (MRI) for diagnosis confirmation, staging and management planning[4].
Treatment options for HCC largely depend on tumor stage, the patient’s functional status and hepatic function reserve. Treatment options range from local-regional treatment (i.e., liver-directed therapies) to surgical options and systemic therapies. Surgical therapies include liver resection or transplantation and are often reserved for eligible patients with early-stage disease. Palliative systemic therapies are typically used in the setting of advanced stage disease, depending on tumor size, presence of distant metastasis and vascular invasion[5]. Immunotherapy checkpoint inhibitors are now considered in many centers the first-line systemic therapies for unresectable HCC, with promising results, particularly when used in combination with other lines of treatment[6,7].
In a significant number of patients, tumor burden is beyond an early stage when surgery would be indicated, but not yet advanced enough to warrant palliation. These patients are therefore treated with local-regional options, either curative, as a bridge to transplant, or downstaging intent. Liver-directed therapies can be considered ablative or catheter-based. Ablative therapies can be energy-based, such as microwave or radiofrequency ablation (RFA), or chemical-based, such as percutaneous ethanol injection. Transcatheter interventions include transarterial chemoembolization (TACE) or radioembolization[8]. With recent technical advances, external beam radiotherapy has also been included in the armamentarium of liver-directed therapies for patients with both early stage or advanced disease. Cost-effectiveness analyses show no significant cost benefit of systemic therapies over Y-90 with respect to quality-adjusted life years[9,10].
Radiation-based local-regional therapies have recently gained wide acceptance due to promising rates of tumor response and safety profiles. Transarterial radioembolization with Yittrium-90 (Y-90) has been shown to be safe and efficacious in local tumor control, with recent data showing superior tumor response rates and survival when compared to TACE[11]. The promising results with Y-90 have prompted the expansion of Y-90 clinical applications to patients with advanced disease, such as patients with HCC tumor in vein[12,13,14]. External beam radiation therapy (EBRT) has also shown an acceptable safety profile and can achieve local tumor control[8,15]. After treatment, assessment of tumor response is crucial for determining management and prognosis. Post-procedure imaging is the primary method for assessing tumor response. While well-established criteria can be applied to assess response to other treatment modalities, predicting HCC response to radiation-based treatment on imaging can be challenging and is still an area of active research.
In this review, we describe radiation-based liver-directed treatment options for HCC, examine current imaging criteria for assessing response to these therapies, discuss practical limitations and gaps in knowledge when applying these response criteria, and address future directions that may help to improve accuracy and outcomes when assessing response to radiation-based HCC treatment.
RADIATION-BASED LOCAL-REGIONAL TREATMENT OF HCC
HCCs are highly vascular neoplasms that preferentially receive blood supply from unpaired hepatic arteries developed during tumor carcinogenesis. The unique reliance on arterial vascularity, in contrast to background liver parenchyma (mostly supplied by the portal vein), enables transcatheter radiation (Y-90) dosage to be selectively delivered to the tumor. Based on vascular selection, treatment can be delivered to the segmental, subsegmental, or lobar level[16]. Conversely, with recent advances in radiation techniques, multidisciplinary guidance and planning, and improved understanding of safe dosing for patients with HCC and cirrhosis, delivery of radiation for HCC treatment can also be done using an external source (EBRT) focusing on a target tissue volume[17].
Transarterial radioembolization with Y-90
Radioembolization with Y-90 may be used as neoadjuvant therapy for patients who are potential transplant candidates, or with a palliative or curative intent based on the patient’s stage of disease[18]. In brief, prior to treatment, arteriography of the hepatic vasculature, celiac and superior mesenteric arteries is performed to map the vascular anatomy, including the assessment of anatomical variants. The initial mapping study assesses the vasculature for excess hepatopulmonary shunting, a contraindication to treatment that could lead to radiation pneumonitis[19]. Similarly, the blood supply to the gastroduodenal region is assessed to avoid the complication of gastric ulceration. Next, through percutaneous, fluoroscopic guidance, Y-90 particles are delivered to the tumor via its vascular supply. Y-90 radiation is delivered via transcatheter approach selectively into the tumor to allow for the highest possible dose through one of two commercially available microsphere particles, glass (TheraSphere; Boston Scientific Corporation, Marlborough, MA) or resin (SIR-Spheres; Sirtex Medical, Sydney, Australia).
A few studies have shown high efficacy and safety of Y-90 as a treatment modality for HCC. When compared to TACE in a phase II trial, Barcelona Clinic Liver Cancer (BCLC) stage A or B patients that underwent Y-90 radioembolization had significantly longer time to progression (> 26 months vs. 6.8 months, P-value: 0.0012), demonstrating superior durability of the Y-90[20]. In a more recent phase II trial comparing TACE to Y90 for use in intermediate-stage HCC, Y-90 treatment conferred a survival benefit of 30.2 months vs. 15.6 months (HR, 0.48; 95%CI: 0.28-0.82; P = .006)[11]. In another study of 207 patients with unresectable HCC, 19% demonstrated downstaging to within the Milan transplant criteria and 82% were bridged to transplant using Y-90. Patients who received a liver transplant had a median recurrence-free survival of 120 months[21]. The safety profile of Y-90 has been well documented in the literature. Complications may be prevented and mitigated by appropriate patient selection, and the incidence of complications requiring intervention is often low, i.e., less than 9%[22]. When complications occur, the clinical nature of the complication and institutional preferences dictate management, which can be performed individually by the interventional radiologist or on a multidisciplinary basis.
External beam radiation
External beam radiation therapy has become increasingly utilized in the treatment of unresectable HCC. The goal of EBRT is to deliver high radiation doses focused on the tumor while sparing adjacent nontumorous liver tissue. While EBRT is most used to slow disease progression in advanced stage HCC, i.e., palliation, a few studies have suggested EBRT as an effective bridge to liver transplantation or curative tumor resection[23].
Advances in technology, such as intensity-modulated radiotherapy or stereotactic body radiotherapy (SBRT), allow for the targeting of small volumes of tissue and have contributed to the increased use of EBRT in patients with chronic liver disease due to reduced nontumorous liver tissue toxicity. Research has been performed to investigate the safety, effectiveness and rates of response of EBRT for HCC treatment. Qui et al. investigated the safety of SBRT for local tumor control in a heterogeneous group of patients ineligible for transplant. In that study, 86% of patients experienced no or mild treatment-related complications with median overall survival of 8.8 months[24]. A meta-analysis by Rim et al. in 2017 compared the safety profile and efficacy of different external radiotherapy modalities in patients with HCC and concurrent portal vein thrombosis. Response rates and 1- and 2-year survival rates were higher for SBRT, with pooled local control rates reaching 86.9% and 2-year survival rates of 26.8%[25].
ASSESSMENT OF RESPONSE TO TREATMENT
After treatment, it is important to assess tumor response to determine further management, patient prognosis, and endpoint outcomes for clinical trials. Histopathologic changes within the post-radiation treatment bed are well characterized[26,27], although histologic assessment of response with follow-up biopsy in clinical practice is not feasible. Imaging, aided or not by clinical and laboratory data (e.g., Alpha-fetoprotein levels), is the most used method to assess changes in tumor burden as a predictor of treatment response.
To standardize imaging interpretation and reporting of HCC response to local-regional treatment, different imaging-based response assessment systems have been validated[28]. Among these systems, two emerge as the most used for clinical care and clinical trials: the Liver Imaging Reporting and Data System (LI-RADS) Treatment Response Algorithm (LR-TRA) and the modified Response Evaluation Criteria in Solid Tumors (mRECIST). While both systems rely on contrast enhancement of any residual viable tumor to quantify the response, the main difference between the two systems relates to the approach to reporting response. LR-TRA assesses response at the individual lesion level, while mRECIST reports response at the patient level.
LR-TRA
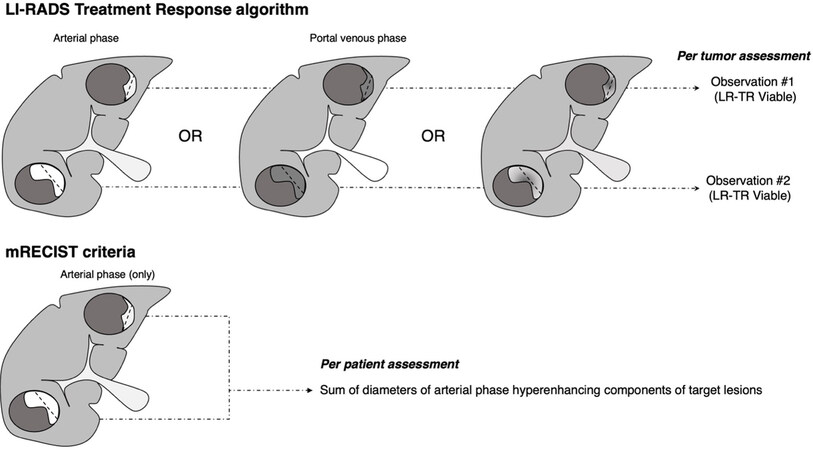
The LR-TRA is designed to assess the response of HCC previously treated with local-regional therapies on contrast-enhanced multiphase CT or MRI[29]. In the LR-TRA system, tumor response is categorized based on the presence or absence of residual arterial phase hyperenhancement (APHE), washout, or enhancement similar to pretreatment imaging. There are three different response categories: LR-TR Nonviable, LR-TR Equivocal, and LR-TR Viable[30]. Residual tumor, if applicable, is measured as the longest unidimensional diameter of the contrast-enhancing component. The LR-TR nonviable category denotes response when tumor demonstrates no contrast enhancement after treatment, or only expected treatment-specific enhancement[28,31]. The LR-TR Viable category indicates the presence of residual viable HCC when tumors demonstrate unequivocal persistent nodular or thick rim arterial phase hyperenhancement, areas of washout, or similar enhancement to the pretreatment imaging findings. The third category, LR-TR Equivocal, is designed to maximize specificity of the LR-TR Viable category and to communicate uncertainty of the response, recommending attention and triggering short-term imaging follow-up. LR-TR Equivocal is assigned when imaging findings are ambiguous, such as enhancement that is not the expected treatment-specific pattern or does not meet the criteria for viability. In a study of 36 patients with 53 HCC lesions, it was shown that 81% of post-TACE HCC tumors that were characterized as LR-TR nonviable demonstrated 100% necrosis on pathology[32]. When imaging studies are deemed inadequate for the assessment of tumor response due to image omissions or excessive motion artifacts, findings are categorized as LR-TR Nonevaluable, an additional LR-TRA category to communicate the need for additional or repeat imaging for assessment of response [Table 1]. Schematic representation of the LR-TRA is shown in Figure 1.
Figure 1. Comparison between Liver Imaging Reporting and Data System (LI-RADS) treatment response algorithm and modified Response Evaluation Criteria in Solid Tumors (mRECIST) to assess response. In the LR-Treatment response algorithm (LR-TRA), viable tumor is characterized by the presence of residual nodular or thick rim arterial phase hyperenhancement, or nodular or thick rim washout, or any enhancement that is similar to the pretreatment tumor characteristics in any imaging phase. In mRECIST, only residual arterial phase hyperenhancement is considered viable tumor. In both systems, residual tumor is measured as the longest diameter of the enhancing component of a treated tumor. In the LR-TRA, response is assessed on a per lesion basis, whereas in mRECIST, response is assessed on a per patient basis.
mRECIST and LT-TRA categories in assessment of treated HCC tumors
| mRECIST | LR-TRA | ||
| Category | Criteria | Category | Criteria |
| Complete Response | No intra-tumoral arterial phase enhancement in all target lesions | LR-TR Nonviable | No residual arterial phase hyperenhancement OR residual washout OR residual enhancement similar to pretreatment; Treatment-specific expected enhancement only |
| Partial Response | ≥ 30% reduction in the sum of diameters of arterial phase hyperenhancing components of target lesions | LR-TR Equivocal | Enhancement that is atypical for treatment specific pattern OR enhancement that does not meet criteria for LR-TR Viable or LR-TR Nonviable |
| Stable disease | Imaging features not categorizable as neither partial response nor progressive disease | LR-TR Viable | APHE or washout that is nodular, masslike or thick irregular rim along the treated observation OR enhancement pattern similar to pretreatment |
| Progressive disease | ≥ 20% increase in the sum of diameters of arterial phase hyperenhancing components of target lesions | ||
| LR-TR Nonevaluable | Post-procedural imaging is limited by artifacts or phase omissions that hinder assessment | ||
Local-regional therapies of HCC are unique in that individual tumors within the same organ can be treated with different modalities or at different time points. To address this point, the LR-TRA assessment is done at the lesion level instead of at the patient level. This not only allows for better management and assessment of specific individual lesions in need of retreatment, but also assesses liver transplant candidacy based on the number and size of viable tumors. Thus, the LR-TRA is potentially better suited for assessing transplant eligibility in patients who underwent downstaging as a bridge to transplantation[17]. Like the LI-RADS diagnostic algorithm, the LR-TRA yields high specificity and positive predictive value for detecting viable tumors on imaging[32,33]. Shorphsire et al. found between an 86% to 96% positive predictive value of the LR-TR viable category for predicting incomplete necrosis on CT or MRI in patients treated with bland arterial embolization among three independent readers[33]. On gadoxetate-enhanced MR images, Kim et al. reported 98% specificity of the LR-TR Viable category for residual viable tumors in patients treated with TACE or RFA, which was significantly higher compared to mRECIST criteria. Conversely, the mRECIST criteria showed significantly higher sensitivity[34].
While most studies evaluating the diagnostic performance of the LR-TRA for local-regional therapies have been performed on cohorts not treated with radiation-based therapies, a few recent studies have demonstrated that the LR-TRA is likely to perform well in predicting complete and incomplete necrosis in HCC treated with radiation-based therapies. In a study investigating the accuracy of LR-TRA for assessing the imaging response of HCC to SBRT, specificity for incomplete tumor necrosis ranged from 71% to 96% using explant pathology as the reference standard[35]. Yoon et al. investigated the diagnostic performance of the LR-TRA in patients treated with transarterial radioembolization (TARE) using surgical resection as the reference standard[36]. In this small cohort study, specificity of the LR-TR Viable category for incomplete tumor necrosis ranged from 93.3% to 100%. Interestingly, reported inter-reader agreement differs markedly between the two studies, with only fair agreement for SBRT among five readers and almost perfect agreement for TARE among three readers (reported kappa statistics: 0.22 vs. 0.81)[35,36]. Studies with larger populations of patients and meta-analyses are needed to further clarify the role, strengths and limitations of the LR-TRA in assessing response to radiation-based therapies for HCC.
Modified RECIST
While historically the response to treatment of solid tumors was assessed based on changes in tumor size (e.g., World Health Organization criteria, RECIST)[29], the necrosis elicited by locoregional therapy of HCCs may not change overall tumor size. Instead, there may only be a change in the amount of viable tumor, which is quantified by the contrast-enhancing component on imaging [Figure 2]. Hence, the modified RECIST criteria (mRECIST) is an iteration of the original RECIST criteria to address this limitation and is applied specifically to assess response to treatment of HCCs[37]. Within this context, HCC tumors that are at least 10 mm in size, nodular, non-infiltrating, with arterial phase enhancement on cross-sectional imaging are considered “target lesions” for response assessment. Non-target lesions are HCC tumors that are less than 10 mm, infiltrating in appearance, or with atypical arterial phase enhancement. Like LR-TRA, mRECIST measures the longest unidimensional diameter of the residual enhancing component (i.e., viable tumor) to quantify response. However, only the presence of arterial phase hyperenhancement is considered viable tumor, not accounting for the presence of washout or other enhancement patterns that are similar to pretreatment imaging[37]. Response is categorized on post-treatment imaging on a per-patient basis as Complete Response (CR), Partial Response (PR), Stable Disease (SD) or Progressive Disease (PD) through the sum change in diameter of the arterial phase hyperenhancing components of the target and non-target lesions, or presence of new lesions fulfilling diagnostic criteria for HCC [Table 1][38]. Given its per patient response assessment, mRECIST is routinely used as endpoint of clinical trials, though its general concept for residual tumor(s) measurement can also be translated into clinical practice. Schematic representation of mRECIST criteria is shown in Figure 1.
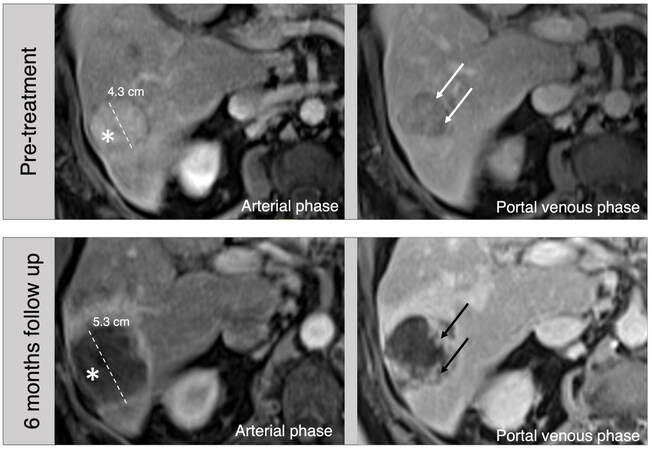
Figure 2. 76-year-old male, hepatitis C cirrhosis, MR images. On pretreatment images, a 4.3 cm arterial phase hyperenhancing (asterisk) observation with portal venous phase washout (arrows) in the right lobe is consistent with HCC (LR-5). Follow-up images 6 months after Y-90 radioembolization shows a larger appearance of the mass due to treatment changes. The lack of arterial phase hyperenhancement (asterisk) and irregular progressive enhancement on portal venous phase (black arrows) are consistent with post-treatment changes (LR-TR Nonviable).
In a study that included 332 patients with intermediate stage HCC, the mRECIST score was an independent predictor for overall survival[39]. Given the per patient assessment, and possibly due to the less stringent imaging criteria (i.e., only arterial phase hyperenhancement is considered viable tumor), mRECIST has been shown to have higher sensitivity at lower specificities for incomplete tumor response. When compared to LR-TRA on gadoxetate-enhanced MR images, mRECIST criteria for viable tumors showed a sensitivity of 93%, with specificity varying from 62%-73%[34]. The inclusion of additional clinical information, however, has been shown to improve the diagnostic performance of mRECIST criteria. Xu et al. found that the positive predictive value of mRECIST was higher for predicting partial necrosis on explant pathology when combined with AFP levels < 20 ng/mL[40]. Historically, mRECIST has been the primary system used to assess response to radiation-based therapies. Larger studies have investigated HCC response to Y-90 therapy, with or without pathology confirmation. In the LEGACY study, mRECIST was used as a study endpoint, showing rates of complete response higher than 80% in follow-up imaging studies[41]. In a study investigating intraprocedural parameters and characteristics associated with complete pathologic response, Toskich et al. found a statistically significant association between increased tumor necrosis and mRECIST response criteria[42].
LIMITATIONS RELATED TO IMAGING ASSESSMENT OF RESPONSE TO RADIATION-BASED THERAPIES
While the use of contrast enhancement characteristics to assess response to ablative and transarterial therapies has been extensively validated[32-34,43], the value of imaging features on contrast-enhanced CT and MRI to predict response following radiation-based therapies such as Y-90 and EBRT is still an active area of research and debate. After radiation, hyperemic alterations in the treatment bed, along with specific patterns of tumor necrosis, can be confounding factors to reliably associate the presence of contrast enhancement with the presence of viable tumor. In a study by Okada et al. 2022, pathologic specimens were evaluated following radiotherapy treatment at multiple time points, ranging from 1.5 to 14 months. Areas surrounding treated HCC were also analyzed histologically. Sinusoidal enlargement with endothelial breakdown and increased arteriole populations were found, which were thought to explain the persistent arterial phase hyperenhancement seen after radiation therapy that challenges the assessment of response on imaging[27].
Persistent hyperenhancement in nonviable areas can cause ambiguous interpretation and may result in limited inter-reader agreement when assessing response[28,35]. Specifically, in transarterial Y-90 therapy, the HCC tumor undergoes necrosis due to both internal radiation and ischemia[44]. There is high energy radiation with low penetration, which selectively targets tumor and generally spares adjacent healthy tissues. Expected benign treatment-related findings include perivascular edema, which is related to distribution of microspheres into the vascular plexus of the tumor, direct radiation effect, leading to increased tissue perfusion and overall changes in the vascular territory of the treated segment, as well as evolving necrosis and tissue scaring[44].
Therefore, the application of existing imaging criteria to assess response in post-radiation HCC treatment warrants caution. The enhancement pattern can be variable, and to date, a decrease in tumor size over time has been shown to be the most reliable predictor of response. Furthermore, it is important to consider that imaging features will evolve over time, and the interval between treatment and follow-up imaging must be considered when assessing response. Persistent arterial phase hyperenhancement can be seen up to 24 months following treatment with SBRT, even in nonviable tumors, and therefore the assessment should be made in combination with changes in size and other clinical or laboratory markers of response
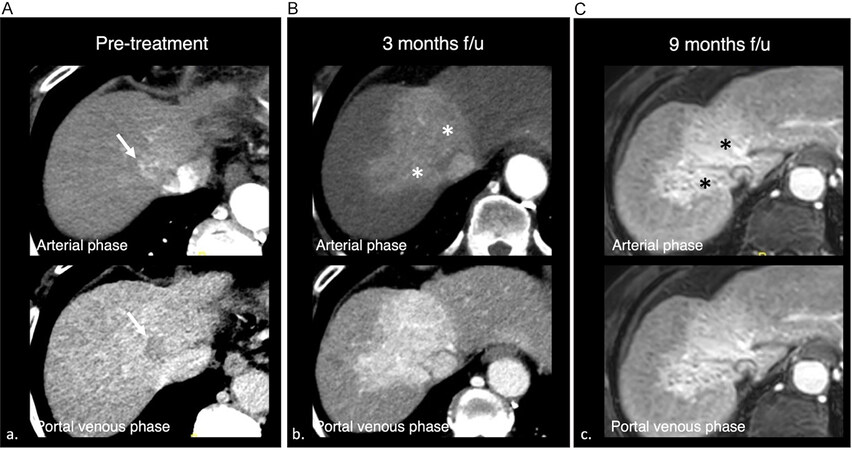
Figure 3. 65-year-old male, cirrhosis complicated by hepatocellular carcinoma. A: Pretreatment computer tomography (CT) shows a 2.2 cm arterial phase hyperenhancing mass in segment 4A/8, with portal venous phase washout, consistent with hepatocellular carcinoma (LR-5). B: Three months CT follow-up after SBRT. Note marked arterial phase hyperenhancement in the treatment zone (asterisks). C: Nine-month magnetic resonance imaging follow-up images show persistent arterial phase hyperenhancement, which may create confusion in the assessment of response, although the decrease in lesion size favors response.
Considering these limitations, mRECIST may not be ideal for the assessment of HCC response to radiation therapies due to its dependence on APHE to define viable disease. For example, a Y-90 treated lesion can show APHE due to hyperemic response, progressive tumor response and granulation tissue[44]. The infiltrative appearance of these potentially benign changes could falsely skew the size of the viable portion of a post-treatment HCC [Figure 4]. Similarly, radiation-based therapies may challenge the application of the LR-TRA as it may overcategorize successfully treated tumors as LR-TR Viable or Equivocal due to residual or diminishing arterial phase hyperenhancement and size [Figure 5]. To address these limitations, active research is needed to identify additional or better predictors of response in the setting of radiation-based therapy that do not rely so heavily on the presence of residual arterial phase hyperenhancement. Working groups within LI-RADS have been actively working to develop a specific response algorithm to radiation-based treatment that incorporates additional imaging features. Currently, a decrease in size in serial imaging is the most reliable predictor of favorable response[45,46]. While an unchanged pattern of enhancement with a stable decrease in tumor size for one year or more can be classified as nonviable due to lack of progression[46], this obscures the clinical picture in the immediate post-treatment phase, when mRECIST stable disease or LR-TR Viable or Equivocal categories may potentially trigger needless additional treatment. As knowledge accrues, residual or diminishing arterial phase hyperenhancement and size seem to be common findings in responding tumors, particularly in the first 12 months following treatment[30]. To address these limitations, changes in the LR-TRA to tailor the response assessment to individual treatment modality groups are likely to be adopted, as additional studies to investigate better predictors of response to radiation-based therapies should be proposed, designed and executed in the near future.
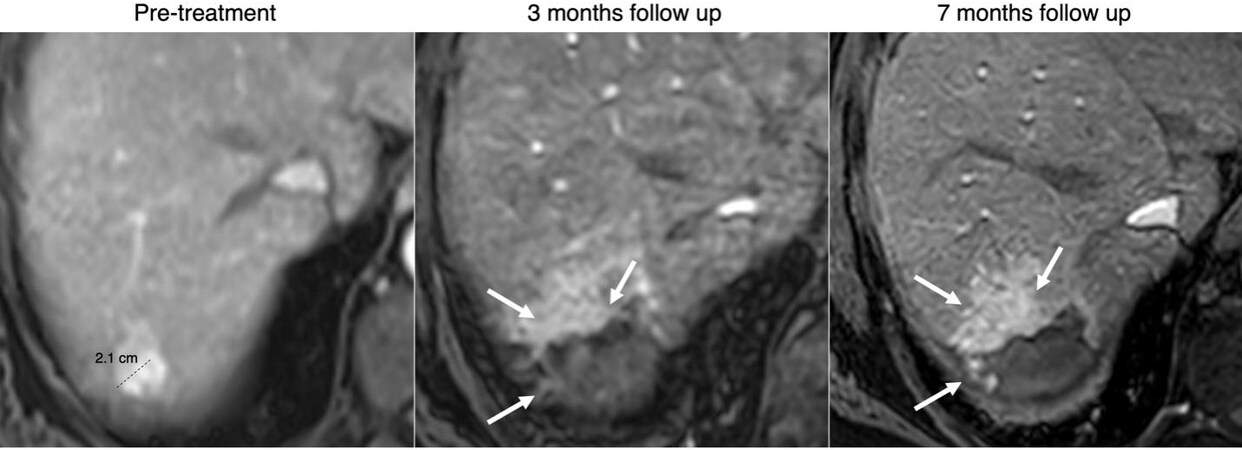
Figure 4. 65-year-old male, hepatitis C cirrhosis, arterial phase MR images. Pretreatment image shows a 2.1 LR-5 observation with APHE and washout (not shown), consistent with HCC. Three- and seven months follow-up image after Y-90 shows peripheral APHE along the treatment zone (arrows), most likely related to hyperemic response, progressive tumor response with granulation tissue and fibrosis development.
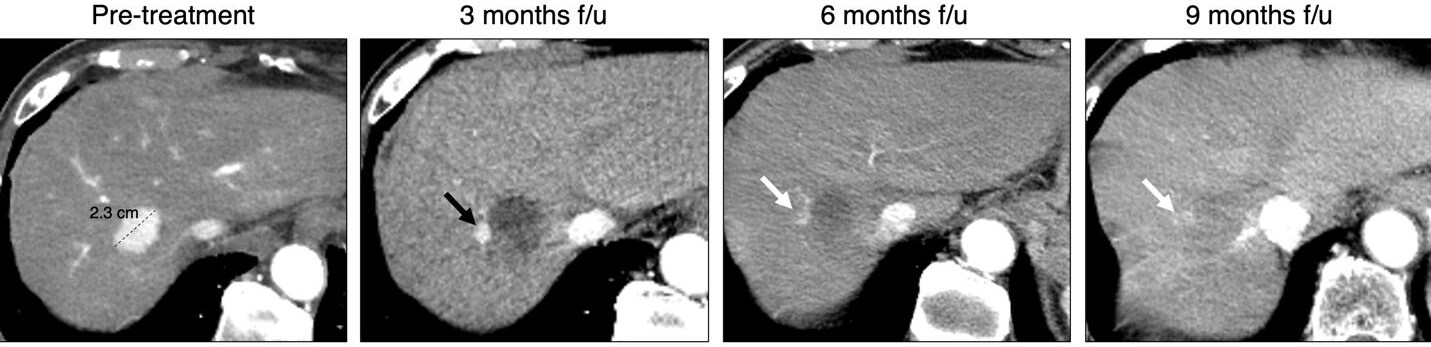
Figure 5. 89-year-old male, chronic hepatitis B infection, arterial phase CT images. Pretreatment image shows a 2.3 LR-5 observation with APHE and washout (not shown), consistent with hepatocellular carcinoma (HCC). Three-month follow-up image after Y-90 shows peripheral nodular arterial phase hyperenhancement (APHE) along the treatment zone (black arrow) measuring 1.1 cm, which fulfills the criteria for residual viable tumor according to LR-Treatment response algorithm (LR-TRA) and mRECIST. On 6 and 9 months follow-up, note progressive decrease in size, consistent with tumor response (white arrows). Ill-defined APHE is still observed on the 9-month follow-up.
Another potential source of ambiguity when assessing HCC response to radiation-based therapies is the development of intra-tumoral calcifications[48]. This finding has been studied in patients with colorectal cancer and hepatic metastases. In a Phase 1 study that used Y-90 microspheres and concomitant chemotherapy, dystrophic calcification was found in pathology following treatment[49]. To less experienced radiologists, dystrophic calcification may give the false appearance of persistent attenuation of a target lesion, or falsely increase the perceived size of persistent, viable tumor.
FUTURE DIRECTIONS
As the use of radiation-based therapies for HCC broadens, solving the conundrum of response assessment becomes imperative to improve patient care. Improvements in the application of current biomarkers or the development of new technologies are needed to increase accuracy in the assessment of tumor response. For the former, a few studies have already been published incorporating additional imaging features to improve sensitivity for residual viable tumor. Currently, the LR-TRA relies on arterial phase enhancement, delayed phase washout, or pretreatment enhancement pattern, while LI-RADS ancillary features are not used to characterize response. Park et al. showed that the sensitivity of the LR-TRA for assessing response to local-regional treatment can be increased without impact on specificity by applying MRI ancillary features to the response criteria[43]. However, this study did not focus on the specific challenges encountered when assessing response to radiation-based therapies. Similarly, diffusion-weighted MRI (DW-MRI) is another imaging modality with potential added value in the assessment of post-radiotherapy HCC patients. While the presence of restricted diffusion is an ancillary feature that favors malignancy in the LI-RADS diagnostic algorithm, it is not currently applied in the LR-TRA. In a study including 29 patients with viable tumor and 35 patients without viable tumor, adding DWI to conventional MR images improved not only diagnostic performance but also interobserver agreement (k = 0.748 vs. 0.450), the latter being a common limiting factor when assessing response to radiation-based therapies[50].
Newer imaging modalities are being investigated to best evaluate response to radiation-based treatment. In 40 patients with 82 target lesions, Altenbernd et al. 2016 evaluated the role of Dual-Energy CT (DECT) in assessing response when compared to standardized response criteria. The authors described improved conspicuity to differentiate residual viable enhancing tumor vs. necrosis when quantifying Iodine Uptake (IU), and the potential to assess perfusion of the viable tumor tissue[51]. Although promising, the small cohort size and lack of pathology correlation warrant caution when interpreting the study results. Other authors have investigated the use of contrast-enhanced ultrasound (CEUS) in detecting and quantifying residual HCC. A study of 59 patients with 59 HCCs evaluated tumor size and vascularity using CEUS before and sequentially after radiotherapy. CEUS demonstrated not only a decrease in tumor size, but a decrease in vascularity over time of tumors shown to have complete response in sequential follow-up studies[52]. Another study by Delaney et al. 2021 specifically evaluated the role of CEUS in predicting response following Y90 treatment[53]. After 2 weeks following treatment, patients with stable or residual disease on MRI were shown to have greater fractional vascularity on CEUS than patients with partial or complete response. While this is a small pilot study, it shines a light on the unique potential of CEUS to assess tumor vascularity and treatment response. Notably, CEUS has been shown to detect changes in tumor perfusion following TARE as early as one week following treatment[54]. This may represent a timely, cost-effective way to predict outcomes and guide treatment. The perfusion aspect of this evolving response is an interesting topic that could potentially improve the assessment in the post-radiation setting. While CT perfusion imaging has not been well studied in radiotherapy patients, there are multiple studies that evaluate its role in post-TACE patients. Studies have shown that changes in CT perfusion parameters in viable tumors correlate with varying responses of HCC to TACE[55,56]. These studies effectively show that the CT perfusion assessment of viable tumor is not affected by TACE treatments. There is a need for further research to evaluate the role of CT perfusion in assessing the viability of post-radiation treated tumors.
Beyond imaging, additional biomarkers can be important for the assessment of response. In patients with known HCC, pretreatment serum biomarkers are useful in establishing a baseline to later assess treatment response. While the most used serum biomarker is Alpha-fetoprotein (AFP), newer biomarkers have also been investigated. For example, angiopoietin 2 (Ang2) and VEGF have been shown to predict survival in patients treated with sorafenib[57], while their role in assessing response to local-regional therapies is not yet clear. Further, new technologies, such as artificial intelligence (AI), can also contribute to better assessing HCC response to treatment. Deep neural networks have an enormous potential for predicting HCC viability or recurrence, given their ability to compute and analyze large amounts of data, including tumor and patient characteristics, clinical information, and multiple serum biomarker levels[58]. While the use of AI tools for the assessment of response to radiation-based therapies has not been widely used, results from studies applying AI frameworks to assess HCC response to other locoregional therapies (e.g., TACE) are promising, showing very high accuracy[59,60]. The ability to combine numerous clinical and imaging variables in a single predictive algorithm would be one of the main strengths of the adoption of AI in the post-treatment setting. Continued research, additional imaging criteria specific to radiation-based therapy, combination of multiple clinical, laboratory and imaging biomarkers, and the adoption of deep learning would compose a favorable landscape that will increase the accuracy of assessment of HCC tumor response to radiation treatment in the near future.
CONCLUSION
Radiation-based liver directed therapies have emerged as effective and safe modalities for treatment of hepatocellular carcinomas, either with curative, as a bridge to transplant or downstaging, or palliative intent. While the clinical applications of these modalities have increased in the last decade due to advances in technology and a better understanding of tumor biology, research is still needed to address some limitations of current imaging criteria for assessing tumor response to these novel techniques.
DECLARATIONS
Authors’ contributionsMade substantial contributions to conception and design of the manuscript: Banda A, Johnson G, Cunha GM.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declare that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. WHO Health Statistics. Cancer. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer [Last accessed on 3 Jan 2023].
2. Balogh J, Victor D 3rd, Asham EH, et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma 2016;3:41-53.
3. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the american association for the study of liver diseases. Hepatology 2018;68:723-50.
4. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6.
5. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80.
6. Rizzo A, Ricci AD, Gadaleta-Caldarola G, Brandi G. First-line immune checkpoint inhibitor-based combinations in unresectable hepatocellular carcinoma: current management and future challenges. Expert Rev Gastroenterol Hepatol 2021;15:1245-51.
7. Jácome AA, Castro ACG, Vasconcelos JPS, et al. Efficacy and safety associated with immune checkpoint inhibitors in unresectable hepatocellular carcinoma: a meta-analysis. JAMA Netw Open 2021;4:e2136128.
8. Sapisochin G, Barry A, Doherty M, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol 2017;67:92-9.
9. Marqueen KE, Kim E, Ang C, Mazumdar M, Buckstein M, Ferket BS. Cost-effectiveness analysis of selective internal radiotherapy with Yttrium-90 versus sorafenib in locally advanced hepatocellular carcinoma. JCO Oncol Pract 2021;17:e266-77.
10. Williams SJ, Rilling WS, White SB. Quality of life and cost considerations: Y-90 radioembolization. Semin Intervent Radiol 2021;38:482-7.
11. Dhondt E, Lambert B, Hermie L, et al. 90Y radioembolization versus drug-eluting bead chemoembolization for unresectable hepatocellular carcinoma: results from the TRACE phase II randomized controlled trial. Radiology 2022;303:699-710.
12. Salem R, Lewandowski R, Roberts C, et al. Use of Yttrium-90 glass microspheres (TheraSphere) for the treatment of unresectable hepatocellular carcinoma in patients with portal vein thrombosis. J Vasc Interv Radiol 2004;15:335-45.
13. Abouchaleh N, Gabr A, Ali R, et al. 90Y radioembolization for locally advanced hepatocellular carcinoma with portal vein thrombosis: long-term outcomes in a 185-patient cohort. J Nucl Med 2018;59:1042-8.
14. Garin E, Tselikas L, Guiu B, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol 2021;6:17-29.
15. Chen CP. Role of external beam radiotherapy in hepatocellular carcinoma. Clin Liver Dis 2020;24:701-17.
16. Saini A, Wallace A, Alzubaidi S, et al. History and evolution of Yttrium-90 radioembolization for hepatocellular carcinoma. J Clin Med 2019;8:55.
17. Kielar A, Fowler KJ, Lewis S, et al. Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm. Abdom Radiol (NY) 2018;43:218-30.
18. Woerner AJ, Johnson GE. Advances in Y-90 radioembolization for the treatment of hepatocellular carcinoma. HR 2022; doi: 10.20517/2394-5079.2021.122.
19. Murthy R, Nunez R, Szklaruk J, et al. Yttrium-90 microsphere therapy for hepatic malignancy: devices, indications, technical considerations, and potential complications. Radiographics 2005;25 Suppl 1:S41-55.
20. Salem R, Gordon AC, Mouli S, et al. Y-90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2016;151:1155-1163.e2.
21. Boston Scientific Clinical Trials. Bridging and downstaging to surgery hepatology: liver transplantation following Y-90 for HCC. Available from: https://www.bostonscientific.com/en-US/medical-specialties/interventional-radiology/interventional-oncology/cancer-therapies-ablation/therasphere/proven-results/bridging-and-downstaging-to-surgery.html [Last accessed on 3 Jan 2023].
23. Gerum S, Jensen AD, Roeder F. Stereotactic body radiation therapy in patients with hepatocellular carcinoma: a mini-review. World J Gastrointest Oncol 2019;11:367-76.
24. Qiu H, Moravan MJ, Milano MT, Usuki KY, Katz AW. SBRT for hepatocellular carcinoma: 8-year experience from a regional transplant center. J Gastrointest Cancer 2018;49:463-9.
25. Rim CH, Kim CY, Yang DS, Yoon WS. Comparison of radiation therapy modalities for hepatocellular carcinoma with portal vein thrombosis: a meta-analysis and systematic review. Radiother Oncol 2018;129:112-22.
26. Montazeri SA, De la Garza-Ramos C, Lewis AR, et al. Hepatocellular carcinoma radiation segmentectomy treatment intensification prior to liver transplantation increases rates of complete pathologic necrosis: an explant analysis of 75 tumors. Eur J Nucl Med Mol Imaging 2022;49:3892-7.
27. Okada M, Numata K, Nihonmatsu H, et al. Pathological appearance of focal liver reactions after radiotherapy for hepatocellular carcinoma. Diagnostics (Basel) 2022;12:1072.
28. Aslam A, Do RKG, Kambadakone A, et al. Hepatocellular carcinoma liver imaging reporting and data systems treatment response assessment: lessons learned and future directions. World J Hepatol 2020;12:738-53.
29. Voizard N, Cerny M, Assad A, et al. Assessment of hepatocellular carcinoma treatment response with LI-RADS: a pictorial review. Insights Imaging 2019;10:121.
30. Ram R, Kampalath R, Shenoy-Bhangle AS, Arora S, Kielar AZ, Mendiratta-Lala M. LI-RADS treatment response lexicon: review, refresh and resolve with emerging data. Abdom Radiol (NY) 2021;46:3549-57.
31. Sato Y, Watanabe H, Sone M, et al. Japan Interventional Radiology in Oncology Study Group-JIVROSG. Tumor response evaluation criteria for HCC (hepatocellular carcinoma) treated using TACE (transcatheter arterial chemoembolization): RECIST (response evaluation criteria in solid tumors) version 1.1 and mRECIST (modified RECIST): JIVROSG-0602. Ups J Med Sci 2013;118:16-22.
32. Chaudhry M, McGinty KA, Mervak B, et al. The LI-RADS version 2018 MRI treatment response algorithm: evaluation of ablated hepatocellular carcinoma. Radiology 2020;294:320-6.
33. Shropshire EL, Chaudhry M, Miller CM, et al. LI-RADS treatment response algorithm: performance and diagnostic accuracy. Radiology 2019;292:226-34.
34. Kim SW, Joo I, Kim HC, et al. LI-RADS treatment response categorization on gadoxetic acid-enhanced MRI: diagnostic performance compared to mRECIST and added value of ancillary features. Eur Radiol 2020;30:2861-70.
35. Mendiratta-Lala M, Aslam A, Maturen KE, et al. LI-RADS treatment response algorithm: performance and diagnostic accuracy with radiologic-pathologic explant correlation in patients with sbrt-treated hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2022;112:704-14.
36. Yoon J, Lee S, Shin J, Kim SS, Kim GM, Won JY. LI-RADS version 2018 treatment response algorithm: diagnostic performance after transarterial radioembolization for hepatocellular carcinoma. Korean J Radiol 2021;22:1279-88.
37. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60.
38. Fournier L, Ammari S, Thiam R, Cuénod CA. Imaging criteria for assessing tumour response: RECIST, mRECIST, Cheson. Diagn Interv Imaging 2014;95:689-703.
39. Shim JH, Lee HC, Kim SO, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? Radiology 2012;262:708-18.
40. Xu M, Fraum T, Garcia-Aroz S, et al. Predictive value of mRECIST in HCC response to the neoadjuvant locoregional therapies and prognosis affected by the peak afp level before liver transplantation. Available from: https://atcmeetingabstracts.com/abstract/predictive-value-of-mrecist-in-hcc-response-to-the-neoadjuvant-locoregional-therapies-and-prognosis-affected-by-the-peak-afp-level-before-liver-transplantation/ [Last accessed on 3 Jan 2023].
41. Salem R, Johnson GE, Kim E, et al. Yttrium-90 radioembolization for the treatment of solitary, unresectable HCC: the LEGACY study. Hepatology 2021;74:2342-52.
42. Toskich B, Vidal LL, Olson MT, et al. Pathologic response of hepatocellular carcinoma treated with yttrium-90 glass microsphere radiation segmentectomy prior to liver transplantation: a validation Study. J Vasc Interv Radiol 2021;32:518-526.e1.
43. Park S, Joo I, Lee DH, et al. Diagnostic performance of LI-RADS treatment response algorithm for hepatocellular carcinoma: adding ancillary features to mri compared with enhancement patterns at CT and MRI. Radiology 2020;296:554-61.
44. Ibrahim SM, Nikolaidis P, Miller FH, et al. Radiologic findings following Y-90 radioembolization for primary liver malignancies. Abdom Imaging 2009;34:566-81.
45. Mendiratta-Lala M, Masch W, Shankar PR, et al. Magnetic resonance imaging evaluation of hepatocellular carcinoma treated with stereotactic body radiation therapy: long term imaging follow-up. Int J Radiat Oncol Biol Phys 2019;103:169-79.
46. Mendiratta-Lala M, Masch WR, Shampain K, et al. MRI assessment of hepatocellular carcinoma after local-regional therapy: a comprehensive review. Radiol Imaging Cancer 2020;2:e190024.
47. Riaz A, Kulik L, Lewandowski RJ, et al. Radiologic-pathologic correlation of hepatocellular carcinoma treated with internal radiation using yttrium-90 microspheres. Hepatology 2009;49:1185-93.
48. Yu MH, Kim YJ, Park HS, Jung SI, Jeon HJ. Imaging patterns of intratumoral calcification in the abdominopelvic cavity. Korean J Radiol 2017;18:323-35.
49. Sharma RA, Van Hazel GA, Morgan B, et al. Radioembolization of liver metastases from colorectal cancer using Yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol 2007;25:1099-106.
50. Park HJ, Kim SH, Jang KM, et al. Added value of diffusion-weighted MRI for evaluating viable tumor of hepatocellular carcinomas treated with radiotherapy in patients with chronic liver disease. AJR Am J Roentgenol 2014;202:92-101.
51. Altenbernd J, Wetter A, Forsting M, Umutlu L. Treatment response after radioembolisation in patients with hepatocellular carcinoma-An evaluation with dual energy computed-tomography. Eur J Radiol Open 2016;3:230-5.
52. Funaoka A, Numata K, Takeda A, et al. Use of contrast-enhanced ultrasound with sonazoid for evaluating the radiotherapy efficacy for hepatocellular carcinoma. Diagnostics (Basel) 2021;11:486.
53. Delaney LJ, Tantawi M, Wessner CE, et al. Predicting long-term hepatocellular carcinoma response to transarterial radioembolization using contrast-enhanced ultrasound: initial experiences. Ultrasound Med Biol 2021;47:2523-31.
54. Eisenbrey JR, Forsberg F, Wessner CE, et al. US-triggered microbubble destruction for augmenting hepatocellular carcinoma response to transarterial radioembolization: a randomized pilot clinical trial. Radiology 2021;298:450-7.
55. Chen G, Ma DQ, He W, Zhang BF, Zhao LQ. Computed tomography perfusion in evaluating the therapeutic effect of transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol 2008;14:5738-43.
56. Ippolito D, Fior D, Bonaffini PA, et al. Quantitative evaluation of CT-perfusion map as indicator of tumor response to transarterial chemoembolization and radiofrequency ablation in HCC patients. Eur J Radiol 2014;83:1665-71.
57. Llovet JM, Peña CE, Lathia CD, Shan M, Meinhardt G, Bruix J. SHARP investigators study group. plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res 2012;18:2290-300.
58. Nam JY, Lee JH, Bae J, et al. Novel model to predict HCC recurrence after liver transplantation obtained using deep learning: a multicenter study. Cancers (Basel) 2020;12:2791.
59. Abajian A, Murali N, Savic LJ, et al. Predicting treatment response to intra-arterial therapies for hepatocellular carcinoma with the use of supervised machine learning-an artificial intelligence concept. J Vasc Interv Radiol 2018;29:850-857.e1.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Banda A, Johnson G, Cunha GM. Assessment of response to liver directed radiation-based therapies: Current guidelines, challenges, and future directions. Hepatoma Res 2023;9:1. http://dx.doi.org/10.20517/2394-5079.2022.60
AMA Style
Banda A, Johnson G, Cunha GM. Assessment of response to liver directed radiation-based therapies: Current guidelines, challenges, and future directions. Hepatoma Research. 2023; 9: 1. http://dx.doi.org/10.20517/2394-5079.2022.60
Chicago/Turabian Style
Banda, Anisha, Guy Johnson, Guilherme Moura Cunha. 2023. "Assessment of response to liver directed radiation-based therapies: Current guidelines, challenges, and future directions" Hepatoma Research. 9: 1. http://dx.doi.org/10.20517/2394-5079.2022.60
ACS Style
Banda, A.; Johnson G.; Cunha GM. Assessment of response to liver directed radiation-based therapies: Current guidelines, challenges, and future directions. Hepatoma. Res. 2023, 9, 1. http://dx.doi.org/10.20517/2394-5079.2022.60
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 9 clicks
Cite This Article 9 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.