Radical skeletal metastatic site irradiation in high-risk neuroblastoma: systematic review and proposal for a randomised trial: a report from the SIOPEN Radiotherapy Committee
Abstract
Aim: Neuroblastoma has a variable outcome depending on age, stage, and molecular pathology. Distant metastatic disease is the central feature of high-risk disease. Recommendations for irradiating persistent metastatic deposits with curative intent after systemic therapy vary. It is unclear to what extent this practice may improve local control or survival. This study systematically reviewed the evidence for skeletal metastatic site irradiation and made evidence-based recommendations for clinical practice.
Methods: We systematically reviewed the literature on radical radiotherapy of persistent metastases after chemotherapy. The aim was to determine whether a position could be taken regarding metastatic site irradiation in combined modality treatment protocols aiming for a cure and whether recommendations could be formulated.
Results: The initial search yielded 445 results. After the title and abstract review, 13 full papers were retrieved. Ten papers were found suitable for data extraction. One additional paper was identified. All 11 were graded as Centre for Evidence-Based Medicine Step 4 in quality; there was no high-level evidence. There are suggestions of benefit for skeletal site irradiation in high-risk neuroblastoma; however, there is no certainty, and it was not possible to recommend a particular treatment policy.
Conclusion: We recommend that consideration is given to a randomised evaluation of the benefits of radiotherapy to a limited number of residual post-induction-chemotherapy metastatic sites in good responders. This practice could be incorporated as an amendment to existing trials.
Keywords
INTRODUCTION
Neuroblastoma is a common childhood cancer, occurring most often in babies and pre-school-age children, less commonly in older children and teenagers, and very rarely in adults[1]. The outcomes vary depending on age at diagnosis, stage at presentation, and molecular pathology features, which are used for risk stratification[2]. High-risk disease is typically defined as Stage M disease[3] occurring in those over 12 or 18 months of age or the presence of MYCN amplification[4]. Distant metastases are usually detected using
Neuroblastoma is a radiosensitive tumour type[18]. Therefore, it is compelling to consider that greater use of radiotherapy might improve outcomes. Given the often great extent of osteo-medullary metastatic sites at the presentation of neuroblastoma, it is not currently considered practical to recommend complete irradiation of all areas involved at the time of diagnosis because of the toxicity it might cause[19,20]. However, total body irradiation was previously considered a practical part of intensification schedules, with high-dose chemotherapy supported by autologous bone marrow transplantation[21]. It has long been recognised that patients with osteo-medullary sites of disease which remain avid on 123I mIBG scintigraphy following induction chemotherapy have worse outcomes than those who clear their metastatic disease quickly[22,23]; therefore, it may seem a logical and more conservative alternative to limit irradiation to these residual mIBG-positive (or FDG PET-avid) sites. However, whether such a strategy genuinely improves outcomes is controversial, and practice regarding local treatment of metastatic sites varies.
The protocol recommendations in the studies run by the International Society of Paediatric Oncology European Neuroblastoma (SIOPEN) clinical research group High-risk Neuroblastoma 1 (HR-NBL1)
This study aims to determine if there is sufficiently strong evidence to produce treatment guidelines or whether further research is indicated. To achieve this objective, we systematically reviewed the literature regarding external beam radiotherapy for metastatic skeletal sites and judged the strength of the evidence presented.
METHODS
Search strategy
A typical systemic review methodology was employed[26], using a predetermined protocol agreed upon by all authors. MEDLINE via PubMed was searched for English-language articles for the thirty years from 1992 to 2021 up to 21 August 2022. A judgement was made that older publications would not be likely helpful, as so much has changed concerning the imaging and pathology techniques used for diagnosis, staging and risk stratification, and the treatment of neuroblastoma. The search terms were (within titles and abstracts) [[“Neuroblastoma” OR “Ganglioneuroblastoma”] NOT “Olfactory”] AND [“Radiation”OR “Radiotherapy”] AND [“Metastatic” OR “Metastases”].
Two authors independently reviewed the titles and abstracts of the search results. They looked for papers of potential relevance about the treatment of metastatic skeletal disease with external beam radiotherapy as part of frontline treatment in International Neuroblastoma Staging System (INSS) stage 4[27] or International Neuroblastoma Risk Group Staging System (INRGSS) stage M[3] neuroblastoma. Exclusion criteria included studies with fewer than ten patients, studies that did not include metastatic skeletal sites given the vast majority of metastases in neuroblastoma are to the bone, studies of molecular radiotherapy in the primary treatment, studies of total body irradiation, studies of palliative treatment, and studies of the treatment of relapsed disease. Only studies where focal external beam radiotherapy was offered to persistent metastatic sites in radical primary treatment were considered. The full-text papers considered potentially relevant to this study were then retrieved for detailed examination. Papers found not to contain relevant information were excluded. Differences between decisions on inclusions were resolved by discussion to achieve consensus to identify only articles with relevant data.
Data extraction and analysis
Data were extracted by one author and verified by another. The Oxford Centre for Evidence-Based Medicine (CEBM) was used to grade the results[28]. For example, concerning treatment benefits, the question would be, “does this intervention help?”, The level of evidence in a single paper is graded Step 1 if it is a systematic review of randomised trials or n-of-1 trials, Step 2 if it is a randomised trial or an observational study with a dramatic effect, Step 3 if it is a non-randomised control cohort or follow-up study, Step 4 if it is a case series, a case-controlled study or a historically controlled study, and Step 5 if it is simply mechanism-based reasoning. The findings were collated qualitatively using the PICO system (Population, Intervention, Comparison and Outcome measure)[29].
Synthesis and formulation of conclusions
All authors examined the extracted data, and the evidence was considered for its reliability. Areas of certainty and uncertainty were defined, and a proposal for further research to address clinically significant areas of the agreement was formulated.
RESULTS
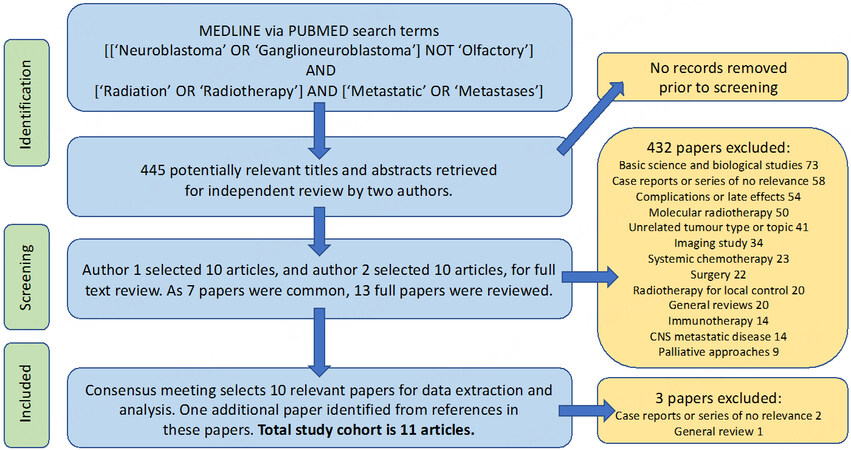
Using our search terms, MEDLINE via PubMed returned 445 results from 1992 to 2021. Of the 445 titles and abstracts reviewed, ten were selected by each author, seven of which had been selected by both authors. The full papers for the 13 articles were reviewed, and a concordance meeting between the authors deemed only ten articles worthy of inclusion for data extraction following review. One additional study, not picked up by the computerised search, was deemed worthy of inclusion in the review of the references of the ten studies. There were, therefore, 11 studies for data extraction. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram in Figure 1 details the principal reasons for exclusions.
No randomised controlled trials were identified. Eleven retrospective studies are detailed here, with a summary of the PICO findings in Table 1.
Population, intervention, comparator and outcome (PICO) summary and centre for evidence-based medicine CEBM level of evidence of the eleven papers identified
| Reference | Population | Intervention | Comparator | Outcome | CEBM |
| Casey et al., 2018[30] | 159 patients, 244 metastatic sites irradiated (166 sites in bone in 150 patients) | Median dose 21 Gy in 14 twice-daily fractions | None | Control 92% in mIBG negative irradiated sites, 62% at positive sites. No difference in local control between bone and other sites | Step 4 |
| Polishchuk et al., 2014[31] | 43 patients total, 13 patients had 19 metastatic sites (18 in bone) irradiated | Radiotherapy typically 21.6 Gy in 14 fractions | Unirradiated metastatic sites | Control reported in 16/19 irradiated sites and 378/506 unirradiated sites | Step 4 |
| Jazmati et al., 2021[32] | 18 patients, 23 metastatic sites irradiated | Median dose 36 Gy (range 20-45 Gy) | None | Control of irradiated metastatic sites 51.4% at 5 years | Step 4 |
| Kushner et al., 2009[33] | 73 patients, 39 patients in complete remission, 34 were poor responders | 21 Gy in 14 twice-daily fractions, photons and electrons (for skull vault) | None | Control of cranial disease 79% in responding patients, 52% in refractory cases | Step 4 |
| Bagley et al., 2019[34] | 11 patients, 16 metastatic sites (not all skeletal) irradiated | Proton therapy, median dose 21.6 Gy Cobalt Grey Equivalent (range 21-24 Gy) | None | No failures observed in irradiated metastatic sites | Step 4 |
| Li et al., 2017[35] | 74 patients, 23 received TBI, 51 did not | Metastatic site irradiation in 19 patients, 25 sites | Relapse rate in patients who did not receive metastatic site irradiation | Relapse rate in irradiated sites 58%, and 75% in non-irradiated sites Relapse rate in TBI patients 52%, and 78% in non-TBI patients | Step 4 |
| Mazloom et al., 2014[36] | 30 patients, 50 metastatic sites (only 14 skeletal) irradiated | 24 Gy in 12 fractions | None | Control rate 74% at 5 years | Step 4 |
| Kandula et al., 2015[37] | 37 patients, 13 underwent metastatic site irradiation | Median dose 21.6 Gy (range 21-30.6 Gy) | Patients without metastatic site irradiation | 77% control in irradiated metastatic sites. No survival differences observed | Step 4 |
| Bradfield et al., 2004[38] | 17 patients | 21 Gy to all metastatic sites identified at diagnosis | None | 4 patients experienced metastatic recurrence – none in an irradiated site | Step 4 |
| Sibley et al., 1995[39] | 26 patients, all received 12 Gy total body irradiation | 13 additionally received 8-24 Gy boost radiotherapy to primary and metastatic sites | 13 patients not receiving a boost | Lower risk of relapse in patients and disease sites receiving boost radiotherapy | Step 4 |
| Sangthawan et al., 2003[40] | 20 patients receiving residual mIBG-positive metastatic site irradiation | 21 Gy in 14 twice-daily fractions | None | 80% control of irradiated sites reported | Step 4 |
The most extensive case study, by Casey et al., detailed the outcomes of 159 patients with a total of 244 irradiated sites (including both bone and non-bone sites) treated with a median dose of 21Gy (in twice-daily 1.5 Gy fractions)[30]. This study showed a local control rate of 92% at irradiated non-persistent metastatic sites post-chemotherapy, similar to their primary site local control rate post gross total resection and 21 Gy (1.5 Gy per fraction), vs. 67% at irradiated persistent sites of disease. No difference was observed in the local control probability between bone and non-bone sites. There was no comparator arm, and the variation in patients and sites made it difficult to assess the value of radiotherapy to these sites. The authors conclude that radiotherapy appears to be an effective modality to achieve local control at metastatic sites.
Polishchuk et al. studied the likelihood of first relapse in 19 irradiated metastatic sites (of which 18 were in bone and one in soft tissue) in 13 patients, compared with the risk of first relapse in 30 patients who had not received metastatic site irradiation[31]. This finding showed that 82.4% of metastatic sites apparent on mIBG scans at first relapse occurred in sites involved at diagnosis. Only a small proportion of sites at relapse were, therefore, new. Relapse occurred in 15.8% of irradiated sites and 25.3% of non-irradiated sites; however, this difference was not statistically significant. Nevertheless, the authors concluded that their observations support the irradiation of metastases that persist after induction chemotherapy.
Jazmati et al. reported eighteen patients presenting with 53 metastatic lesions who received irradiation to 23 mIBG-avid metastatic sites (one to three lesions per patient) to a median dose of 36 Gy (20-45 Gy)[32]. The median follow-up was 149 months. The documented local recurrence-free survival to irradiated metastatic sites was 51.4% at five years, with nine of 18 patients developing “local failure”. This small sample size without a comparator makes this study difficult to conclude. It is challenging to determine who was eligible for irradiation to sites based on what criteria. The role of radiotherapy in this instance remains unclear. The study provides information on the delineation of Planning Target Volume and how it differs amongst different centres, be it delineated on the baseline post-induction chemotherapy volume. The authors concluded that studies with larger cohorts or, ideally, prospective trials would be desirable to elucidate the role of radiotherapy for metastases.
A single-institution study by Kushner et al. sought to reduce the morbidity of metastatic disease in the skull by incorporating mixed photon and electron (to reduce brain irradiation) radiotherapy into the radical treatment strategy[33]. Seventy-three patients (39 in first complete, or very good partial, remission following induction chemotherapy, and 34 with refractory disease) received 21Gy in 14 fractions delivered twice daily. Calvarial sites were irradiated in 45 patients, orbital sites in 26 patients, and the mandible in nine patients. Local control in irradiated sites at three years in the complete and very good partial remission cohort was 79%, and in the refractory group was 52%. The authors described these results as “encouraging” but conceded that a randomised trial might be necessary to prove a disease control advantage. This study came from the same institution as one other[30], and the reported periods overlapped. Therefore, it is possible that some patients were common to both papers; therefore, the two papers cannot be regarded as fully independent.
The use of charged particles in this disease was assessed in one study by Bagley et al., looking at proton use (passive scatter and intensity modulated proton beam therapy) delivered to primary and up to three mIBG-avid metastatic sites after induction chemotherapy and autologous stem cell transplant[34]. Median radiation doses to the primary and metastatic sites were 21.6 Gy (RBE) (range, 21-24 Gy) with a median follow-up of 60.2 months and a minimum follow-up of 12 months. Sixteen sites were irradiated (one site in seven patients, two in three patients, and three in one patient). Two-thirds of the metastatic sites were bone, but visceral and nodal sites were also included in this study. No local failures were observed among the sixteen sites. As this study comprised a heterogeneous patient population treated in various ways, and the numbers were small with no control group, it is challenging to draw firm conclusions about the extent to which radiation treatment may have improved patient outcomes.
Li et al. sought to assess the pattern of relapse on metastatic sites treated with focal external beam radiotherapy by comparing patients who had or had not received prior total body irradiation (TBI)[35]. The cohort comprised 74 patients, of whom 23 underwent TBI. Focal radiotherapy was given to sites of metastatic disease that remained mIBG-positive in 19 patients following induction chemotherapy. Of these, four received TBI, and 15 did not. There were 227 sites of metastatic relapse in the 74 patients, of which 68% occurred in sites of metastatic disease at presentation. Relapse occurred in 58% of the 19 patients who had received focal radiotherapy for residual mIBG-positive lesions and 75% of the 55 patients who had no residual mIBG-positive sites and, therefore, no focal radiotherapy. That difference was not statistically significant. Of 23 patients treated with TBI, 52% relapsed in previously mIBG-avid sites, whereas 78% treated without TBI relapsed in previously mIBG-avid sites. This difference was statistically significant. The authors believe their findings prompt the question of whether an expanded radiotherapy role in metastases will improve disease control.
Mazloom et al. looked at 24 Gy in 12 fractions to metastatic sites following induction therapy to 50 metastatic sites in 30 patients, demonstrating a local control rate of 74% at five years[36]. A maximum of three metastatic sites were irradiated. Only the three most avid sites were treated if patients had a more significant number of metastatic sites visible on the post-induction mIBG scan. The number of mIBG-avid sites present after induction chemotherapy and surgery predicted progression-free and overall survival. Only fourteen irradiated metastases were in bone (the most common metastatic site), and the number of patients was small in this observational study. There was no comparator group, making it challenging to conclude the value of treatment.
A single-institution study by Kandula et al. included 13 patients who received external beam radiotherapy to one metastatic site per patient concurrently with primary site radiotherapy to 24 patients not receiving any radiotherapy to metastatic sites[37]. The median radiotherapy dose was 21.6 Gy (range, 21-30.6 Gy). Three of the 13 patients developed an in-field relapse. There was no significant difference in overall survival or relapse-free survival between the irradiated group and those without metastatic site radiotherapy. The authors concluded that the results from this series require more extensive studies to elucidate the optimal role of definitive radiotherapy in metastatic sites.
In a single-centre study by Bradfield et al., 17 patients received metastatic site irradiation[38]. The intention was that all initial sites of metastatic bone disease would be irradiated to a dose of 21 Gy in 14 daily fractions. However, if the number and extent of bony metastases precluded the inclusion of all initially involved lesions, priority was given to sites of residual abnormality after induction chemotherapy. Only one patient relapsed at an irradiated metastatic site, whereas six relapsed at distant unirradiated sites. The authors conclude that radiotherapy appears effective, although drawing firm conclusions in such a small series is challenging.
In a single-institution study, Sibley et al. studied 26 patients who underwent high-dose chemotherapy with TBI followed by bone marrow transplant[39]. In 13 patients, according to physician judgement, a radiotherapy boost (focal administration of radiotherapy over and above the TBI) was administered to the primary site and metastatic sites where feasible. Six patients were not evaluable because of treatment-related mortality. Of the 20 surviving patients, ten received a boost, and there were three failures; ten did not, among whom there were six failures. An analysis of 38 anatomical sites deemed amenable to a radiotherapy boost showed recurrence in one of ten treated sites and six of 28 unirradiated sites. While the observed differences are not statistically significant, the authors conclude that their data suggest that using radiotherapy may be beneficial.
Finally, a small study by Sangthawan et al. was reviewed; it was not found in our primary literature search but was identified from a prior systematic review[40]. This study evaluated 20 patients who received radiation to residual mIBG-positive sites of disease. Relapses were reported in irradiated sites in four patients and at seven unirradiated sites. The authors noted that the skull is a frequent site of relapse.
All studies are all rated CEBM Step 4. To define the treatment benefits and answer the question “does this intervention help”? the level of evidence we discovered was based on case series, case-control studies, or historically controlled studies. No randomised trials attempted to define the value of systematic irradiation of metastatic sites.
DISCUSSION
A recent European review of the role of radical radiotherapy of metastatic disease across a range of childhood cancers, and a survey of paediatric radiation oncologists, produced some compelling observations[41]. Radiation of at least limited metastatic disease where feasible is recommended in European clinical trials relating to rhabdomyosarcoma, non-rhabdomyosarcoma soft tissue sarcoma, Ewing sarcoma, and Wilms tumour, but not neuroblastoma. Treatment and clinical trial protocols are written by different and independent disease specialist groups, which may explain this difference. Paediatric radiation oncologists expressed concerns about the absence of clearly defined treatment protocols for metastatic disease across different tumour types, as the guidance is often vague. All wished cooperative research groups to conduct randomised trials to determine the actual value of metastatic site irradiation. The European paediatric Soft-tissue Sarcoma Group’s Frontline and Relapsed Rhabdomyosarcoma study
This systematic review did not find any high-quality evidence, such as randomised trials, answering the question of the actual value of systematic irradiation of metastatic skeletal sites in high-risk neuroblastoma as part of primary treatment. All the publications contained a relatively low level of evidence. No investigator applied the most appropriate analytical methods to the question. These studies were all retrospective audits of clinical outcomes rather than carefully planned prospective research. The criteria for giving radiotherapy to metastatic sites were not stated or perhaps attributed to “physician choice”, which may introduce bias. Even within some individual papers, the dose/fractionation schedules varied or were sometimes unknown or not stated. Only three of the 11 studies reported more than 50 irradiated patients, and seven reported only 20 or fewer. While there is certainly a suggestion that irradiation of metastatic skeletal sites may confer a benefit, most studies contained too few patients to be confident that the observations would be the same in a larger patient group. It is, therefore, not possible, based on this review, to give definite recommendations regarding the use of radiotherapy for metastatic skeletal sites in high-risk neuroblastoma. Most authors indicated that their findings pointed towards the need for further prospective studies to answer the question.
There are several limitations which make the interpretation of the literature challenging. The methodology reported in the various papers cited was heterogeneous, making direct comparisons difficult. While broadly similar in principle in different parts of the world, treatment protocols differ in detail and have changed over time. There may therefore be confounding variables present but not always apparent. Most papers give data on patients treated before the current anti-GD2 immunotherapy era, and it is possible that as this new systemic treatment has improved outcomes[12,13], the value of skeletal metastatic site irradiation may have changed. The effect of variations in other systemic therapies used, for example, high-dose chemotherapy[10], double high-dose chemotherapy schedules (tandem transplant)[11], 131I mIBG therapy in induction[42], or anaplastic lymphoma kinase inhibitors[43], is unknown. Any new trial should be properly stratified to consider these variables.
We believe that only a carefully designed and adequately powered randomised trial will provide a definitive answer to the question, “does systematic irradiation of metastatic skeletal sites improve the outcome of patients treated with contemporary protocols for high-risk neuroblastoma?” In such a trial, the potential confounding variables will be standardised due to the stratification required for randomisation. There is an ethical imperative to conduct a trial of this sort, as equipoise exists in the paediatric oncology community[44]. If this intervention genuinely adds value, it is wrong to withhold it; on the other hand, if it adds only cost and toxicity[20,45], it is wrong to use it.
As treatment of all involved metastatic sites at presentation would often be impractical (because the number and extent of involved skeletal segments are usually high), we recommend that the trial explores the value of treating only post-induction chemotherapy mIBG-positive disease sites. As in SIOPEN studies, patients who, after completion of induction chemotherapy, have more than three spots visible on mIBG scan reassessment are deemed poor responders and are offered alternative salvage treatments either electively or within another clinical trial. We propose to limit eligibility to the randomised trial to those patients who have responded well enough to continue to high-dose chemotherapy and have between one and three mIBG scan-positive sites of residual disease.
Data from a randomisation comparing the efficacy of two induction regimens in the SIOPEN High-Risk Neuroblastoma Trial have been published[9]. As there was no significant difference in efficacy between the two arms of the trial, results from the combined patient population are quoted here. Overall, 59% had an adequate response after induction chemotherapy to proceed to high-dose chemotherapy, and skeletal evaluation by mIBG scintigraphy showed a complete metastatic response in 36%. These figures suggest that 23%, approximately one-quarter of the high-risk patient population, might be eligible for such a trial.
The most valid result would be an improvement in overall survival, and so we propose this as the primary endpoint of a future trial, although an increase in metastatic site control or prolongation in time to progression (event-free survival) would also be worthwhile outcomes, and so could be secondary outcome measures. The proposed study would probably not require a new trial: it is possible that an amendment could be made to existing high-risk neuroblastoma trials.
DECLARATIONS
Authors’ contributionsDesign: Gaze MN, Boterberg T, Dieckmann K
Literature research, data analysis, manuscript writing: Keshwani K, Gaze MN
Manuscript editing, final approval: All authors
Availability of data and materialsData were obtained from MEDLINE via PubMed.
Financial support and sponsorshipDr. Gaze MN is supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre and the Radiation Research Unit at the Cancer Research UK City of London Centre Award [C7893/A28990].
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
2. Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 2009;27:289-97.
3. Monclair T, Brodeur GM, Ambros PF, et al. The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. J Clin Oncol 2009;27:298-303.
4. Ambros PF, Ambros IM, Brodeur GM, et al. International consensus for neuroblastoma molecular diagnostics: report from the International Neuroblastoma Risk Group (INRG) Biology Committee. Br J Cancer 2009;100:1471-82.
5. Matthay KK, Shulkin B, Ladenstein R, et al. Criteria for evaluation of disease extent by (123)I-metaiodobenzylguanidine scans in neuroblastoma: a report for the International Neuroblastoma Risk Group (INRG) Task Force. Br J Cancer 2010;102:1319-26.
6. Sung AJ, Weiss BD, Sharp SE, Zhang B, Trout AT. Prognostic significance of pretreatment 18F-FDG positron emission tomography/computed tomography in pediatric neuroblastoma. Pediatr Radiol 2021;51:1400-5.
7. Berlanga P, Pasqualini C, Pötschger U, et al. Central nervous system relapse in high-risk stage 4 neuroblastoma: The HR-NBL1/SIOPEN trial experience. Eur J Cancer 2021;144:1-8.
8. Ladenstein R, Lambert B, Pötschger U, et al. Validation of the mIBG skeletal SIOPEN scoring method in two independent high-risk neuroblastoma populations: the SIOPEN/HR-NBL1 and COG-A3973 trials. Eur J Nucl Med Mol Imaging 2018;45:292-305.
9. Garaventa A, Poetschger U, Valteau-Couanet D, et al. Randomized trial of two induction therapy regimens for high-risk neuroblastoma: HR-NBL1.5 International society of pediatric oncology European neuroblastoma group study. J Clin Oncol 2021;39:2552-63.
10. Ladenstein R, Pötschger U, Pearson ADJ, et al. Busulfan and melphalan
11. Park JR, Kreissman SG, London WB, et al. Effect of tandem autologous stem cell transplant vs single transplant on event-free survival in patients with high-risk neuroblastoma: a randomized clinical trial. JAMA 2019;322:746-55.
12. Ladenstein R, Pötschger U, Valteau-Couanet D, et al. Investigation of the role of dinutuximab beta-based immunotherapy in the SIOPEN high-risk neuroblastoma 1 Trial (HR-NBL1). Cancers 2020;12:309.
13. Yu AL, Gilman AL, Ozkaynak MF, et al. Long-term follow-up of a phase III study of ch14.18 (Dinutuximab) + cytokine immunotherapy in children with high-risk neuroblastoma: COG study ANBL0032. Clin Cancer Res 2021;27:2179-89.
14. Holmes K, Pötschger U, Pearson ADJ, et al. Influence of surgical excision on the survival of patients with stage 4 high-risk neuroblastoma: a report from the HR-NBL1/SIOPEN study. J Clin Oncol 2020;38:2902-15.
15. von Allmen D, Davidoff AM, London WB, et al. Impact of extent of resection on local control and survival in patients from the COG A3973 study with high-risk neuroblastoma. J Clin Oncol 2017;35:208-16.
16. Arumugam S, Manning-Cork NJ, Gains JE, Boterberg T, Gaze MN. The evidence for external beam radiotherapy in high-risk neuroblastoma of childhood: a systematic review. Clin Oncol 2019;31:182-90.
17. Liu KX, Naranjo A, Zhang FF, et al. Prospective evaluation of radiation dose escalation in patients with high-risk neuroblastoma and gross residual disease after surgery: a report from the children’s oncology group ANBL0532 study. J Clin Oncol 2020;38:2741-52.
18. Deacon JM, Wilson PA, Peckham MJ. The radiobiology of human neuroblastoma. Radiother Oncol 1985;3:201-9.
19. Flandin I, Hartmann O, Michon J, et al. Impact of TBI on late effects in children treated by megatherapy for Stage IV neuroblastoma. A study of the French Society of Pediatric oncology. Int J Radiat Oncol Biol Phys 2006;64:1424-31.
20. Hobbie WL, Li Y, Carlson C, et al. Late effects in survivors of high-risk neuroblastoma following stem cell transplant with and without total body irradiation. Pediatr Blood Cancer 2022;69:e29537.
21. Haas-Kogan DA, Swift PS, Selch M, et al. Impact of radiotherapy for high-risk neuroblastoma: a Children’s Cancer Group study. Int J Radiat Oncol Biol Phys 2003;56:28-39.
22. Matthay KK, Edeline V, Lumbroso J, et al. Correlation of early metastatic response by 123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J Clin Oncol 2003;21:2486-91.
23. Schmidt M, Simon T, Hero B, Schicha H, Berthold F. The prognostic impact of functional imaging with (123)I-mIBG in patients with stage 4 neuroblastoma >1 year of age on a high-risk treatment protocol: results of the German Neuroblastoma Trial NB97. Eur J Cancer 2008;44:1552-8.
24. Gaze MN, Boterberg T, Dieckmann K, et al. Results of a quality assurance review of external beam radiation therapy in the International Society of Paediatric Oncology (Europe) Neuroblastoma Group’s High-risk Neuroblastoma Trial: a SIOPEN study. Int J Radiat Oncol Biol Phys 2013;85:170-4.
25. Simon T, Hero B, Schulte JH, et al. 2017 GPOH guidelines for diagnosis and treatment of patients with neuroblastic tumors. Klin Padiatr 2017;229:147-67.
26. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1.
27. Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 1993;11:1466-77.
28. Centre for evidence-based medicine: critical appraisal tools. Available from: https://www.cebm.ox.ac.uk/resources/ebm-tools/critical-appraisal-tools [Last accessed on 10 Feb 2023].
29. Centre for evidence-based medicine: finding the evidence: a how-to guide. Available from: https://www.cebm.ox.ac.uk/resources/ebm-tools/finding-the-evidence-tutorial [Last accessed on 10 Feb 2023].
30. Casey DL, Pitter KL, Kushner BH, et al. Radiation therapy to sites of metastatic disease as part of consolidation in high-risk neuroblastoma: can long-term control be achieved? Int J Radiat Oncol Biol Phys 2018;100:1204-9.
31. Polishchuk AL, Li R, Hill-Kayser C, et al. Likelihood of bone recurrence in prior sites of metastasis in patients with high-risk neuroblastoma. Int J Radiat Oncol Biol Phys 2014;89:839-45.
32. Jazmati D, Butzer S, Hero B, et al. Long-term follow-up of children with neuroblastoma receiving radiotherapy to metastatic lesions within the German Neuroblastoma Trials NB97 and NB 2004. Strahlenther Onkol 2021;197:683-9.
33. Kushner BH, Cheung NK, Barker CA, et al. Hyperfractionated low-dose (21 Gy) radiotherapy for cranial skeletal metastases in patients with high-risk neuroblastoma. Int J Radiat Oncol Biol Phys 2009;75:1181-6.
34. Bagley AF, Grosshans DR, Philip NV, et al. Efficacy of proton therapy in children with high-risk and locally recurrent neuroblastoma. Pediatr Blood Cancer 2019;66:e27786.
35. Li R, Polishchuk A, DuBois S, et al. Patterns of relapse in high-risk neuroblastoma patients treated with and without total body irradiation. Int J Radiat Oncol Biol Phys 2017;97:270-7.
36. Mazloom A, Louis CU, Nuchtern J, et al. Radiation therapy to the primary and postinduction chemotherapy MIBG-avid sites in high-risk neuroblastoma. Int J Radiat Oncol Biol Phys 2014;90:858-62.
37. Kandula S, Prabhu RS, Nanda R, et al. Outcomes after radiation therapy to metastatic sites in patients with stage 4 neuroblastoma. J Pediatr Hematol Oncol 2015;37:175-80.
38. Bradfield SM, Douglas JG, Hawkins DS, Sanders JE, Park JR. Fractionated low-dose radiotherapy after myeloablative stem cell transplantation for local control in patients with high-risk neuroblastoma. Cancer 2004;100:1268-75.
39. Sibley GS, Mundt AJ, Goldman S, et al. Patterns of failure following total body irradiation and bone marrow transplantation with or without a radiotherapy boost for advanced neuroblastoma. Int J Radiat Oncol Biol Phys 1995;32:1127-35.
40. Sangthawan D, DesRosiers PM, Randall ME, Robertson K, Goebel S, Fallon R. Relapse in the skull after myeloablative therapy for high-risk neuroblastoma. Pediatr Hematol Oncol 2003;20:23-30.
41. Huijskens SC, Kroon PS, Gaze MN, et al. Radical radiotherapy for paediatric solid tumour metastases: An overview of current European protocols and outcomes of a SIOPE multicenter survey. Eur J Cancer 2021;145:121-31.
42. Kraal KC, Bleeker GM, van Eck-Smit BL, et al. Feasibility, toxicity and response of upfront metaiodobenzylguanidine therapy therapy followed by German Pediatric Oncology Group Neuroblastoma 2004 protocol in newly diagnosed stage 4 neuroblastoma patients. Eur J Cancer 2017;76:188-96.
43. Foster JH, Voss SD, Hall DC, et al. Activity of crizotinib in patients with ALK-aberrant relapsed/refractory neuroblastoma: a children’s oncology group study (ADVL0912). Clin Cancer Res 2021;27:3543-8.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Keshwani K, Boterberg T, Dieckmann K, Janssens GO, Laprie A, Timmermann B, Gaze MN. Radical skeletal metastatic site irradiation in high-risk neuroblastoma: systematic review and proposal for a randomised trial: a report from the SIOPEN Radiotherapy Committee. J Cancer Metastasis Treat 2023;9:1. http://dx.doi.org/10.20517/2394-4722.2022.32
AMA Style
Keshwani K, Boterberg T, Dieckmann K, Janssens GO, Laprie A, Timmermann B, Gaze MN. Radical skeletal metastatic site irradiation in high-risk neuroblastoma: systematic review and proposal for a randomised trial: a report from the SIOPEN Radiotherapy Committee. Journal of Cancer Metastasis and Treatment. 2023; 9(1): 1. http://dx.doi.org/10.20517/2394-4722.2022.32
Chicago/Turabian Style
Keshwani, Karimali, Tom Boterberg, Karin Dieckmann, Geert O. Janssens, Anne Laprie, Beate Timmermann, Mark N. Gaze. 2023. "Radical skeletal metastatic site irradiation in high-risk neuroblastoma: systematic review and proposal for a randomised trial: a report from the SIOPEN Radiotherapy Committee" Journal of Cancer Metastasis and Treatment. 9, no.1: 1. http://dx.doi.org/10.20517/2394-4722.2022.32
ACS Style
Keshwani, K.; Boterberg T.; Dieckmann K.; Janssens GO.; Laprie A.; Timmermann B.; Gaze MN. Radical skeletal metastatic site irradiation in high-risk neuroblastoma: systematic review and proposal for a randomised trial: a report from the SIOPEN Radiotherapy Committee. J. Cancer. Metastasis. Treat. 2023, 9, 1. http://dx.doi.org/10.20517/2394-4722.2022.32
About This Article
Copyright
Data & Comments
Data

 Cite This Article 10 clicks
Cite This Article 10 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.