Endocrine therapy in metastatic breast cancer-more than just CDK4/6 inhibitors
Abstract
Advanced hormone receptor-positive breast cancer is one of the women’s most common malignant diseases and remains incurable despite recent therapeutic innovations. The dependence of hormone receptor-positive breast cancer on hormonal growth signals offers the possibility of inhibiting this signaling pathway using anti-hormonal therapy. Nevertheless, the development of resistance to antitumoral drugs remains a challenge. Molecularly-targeted substances significantly improve survival rates and (as in the case of cyclin-dependent kinase 4 and 6 inhibitors) are widely used in clinical practice and enhance endocrine therapy’s efficacy. Agents such as everolimus, alpelisib, and capivasertib target the phosphoinositide 3 kinase/protein kinase B/mammalian target of rapamycin pathway, which is a promising approach to overcoming endocrine resistance. Novel therapies are being studied in numerous trials, and some already show significant benefits in survival rates. The development of new therapies to avert endocrine resistance is an urgent challenge in modern medicine. The following review will examine some promising therapeutic approaches.
Keywords
INTRODUCTION
Breast cancer is the most commonly diagnosed type of cancer and the leading cause of cancer deaths in women worldwide[1]. The hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative subtype accounts for approximately 70% of cases[2]. Endocrine-based therapeutic strategies are the treatment of choice for this subtype unless there is a visceral crisis or other contraindication. Standard endocrine therapy for postmenopausal women includes selective estrogen receptor modulators (SERMs), selective estrogen receptor degraders (SERDs), non-steroidal aromatase inhibitors (NSAIs), and steroidal aromatase inhibitors[3]. The treatment algorithm has changed substantially since adding cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors to endocrine therapy showed significant outcome benefits. Nevertheless, resistance to endocrine therapies remains a persistent problem. Recent studies identified several mechanisms that lead to resistance to endocrine therapy; these studies identify novel therapeutic approaches that overcome resistance and improve efficacy.
Standard of care: CDK4/6 inhibition
Activation of CDK4/6 is central to regulating cell cycle progression and proliferation driven by estrogen[4,5]. The advent of CDK4/6 inhibitors targeting overactive CDK complexes has transformed the management of HR-positive and HER2-negative metastatic breast cancer. Three CDK4/6 inhibitors (palbociclib, ribociclib, abemaciclib) combined with endocrine therapy demonstrated meaningful clinical benefits[6-17]. Compared to standard chemotherapy, these combination therapies showed similar efficacy with a superior toxicity profile[6,7]. The phase III PEARL trial compared palbociclib plus endocrine therapy with capecitabine in postmenopausal women with aromatase inhibitor (AI)-resistant metastatic breast cancer to investigate differences in progression-free survival and safety profiles[6]. Palbociclib was combined with exemestane (cohort 1) or fulvestrant (cohort 2) and estrogen receptor 1 (ESR1) mutation status was assessed to prevent confounding by acquired endocrine resistance. There was no superiority of palbociclib plus endocrine therapy over capecitabine in both cohorts regarding progression-free survival[6]. On the other hand, significantly more high-grade adverse events were observed with capecitabine than with palbociclib plus endocrine therapy [Table 1]. As a consequence, the rate of treatment discontinuation was twice as high in the capecitabine arm[6].
Summary of the most frequently reported adverse events of grade ≥ 3[6]
| Palbociclib plus exemestane n (%) n = 150 | Palbociclib plus fulvestrant n (%) n = 149 | Capecitabine n (%) n = 289 | |
| Neutropenia | 86 (57.4) | 83 (55.7) | 16 (5.5) |
| Febrile neutropenia | 2 (1.3) | 1 (0.7) | 4 (1.4) |
| Hand/foot syndrome | 0 | 0 | 68 (23.5) |
| Diarrhea | 2 (1.3) | 2 (1.3) | 22 (7.6) |
| Fatigue | 2 (1.3) | 1 (0.7) | 16 (5.5) |
| Anemia | 1 (0.7) | 3 (2.0) | 10 (3.5) |
| SAEs | 24 (16.0) | 19 (12.8) | 63 (21.8) |
| SAEs related to therapy | 6 (4.0) | 5 (3.4) | 30 (10.4) |
One of the first promising signals of CDK4/6 inhibition came from the PALOMA-1/TRIO-18 trial published in 2015[8]. This phase II study showed that the addition of palbociclib to letrozole compared to letrozole alone significantly prolonged progression-free survival [20.2 months vs. 10.2 months; hazard ratio (HR) 0.488; P = 0.0004][8]. Since then, several phase III studies demonstrated the superiority of the addition of a CDK4/6 inhibitor to endocrine therapy compared to endocrine therapy alone regarding progression-free survival in first-line therapy for advanced HR-positive, HER2-negative breast cancer [Table 2][8-10,12,18].
Selected trials investigating CDK4/6 inhibitors as first-line therapy
| Trial | n | Agent | Median PFS (mo.) | HR [95%CI] | Median OS (mo.) | HR [95%CI] |
| PALOMA-1 (phase II trial)[8,135] | 165 | Palbociclib + letrozole vs. letrozole | 20.2 vs. 10.2 | 0.488 [0.319- 0.748] | 37.5 vs. 34.5 | 0.897 [0.623-1.294] |
| PALOMA-2 (phase III trial)[20,21] | 666 | Palbociclib + letrozole vs. letrozole | 27.6 vs. 14.5 | 0.563 [0.461-0.687] | 53.9 vs. 51.2 | 0.956 [0.777-1.177] |
| MONALEESA-2 (phase III trial)[11,18] | 668 | Ribociclib + letrozole vs. letrozole | 25.3 vs. 16.0 | 0.568 [0.457-0.704] | 63.9 vs. 51.4 | 0.76 [0.63-0.93] |
| MONALEESA-7 (phase III trial)a[10,14] | 672 | Ribociclib + tamoxifen/NSAI vs. tamoxifen/NSAI All + GnRH | 23.8 vs. 13.0 | 0.55 [0.44-0.69] | n.r. vs. 40.9 | [37.8-n.r.] |
| MONARCH-3 (phase III trial)[12] | 493 | Abemaciclib + NSAI vs. NSAI | 28.18 vs. 14.76 | 0.54 [0.418-0.698] | - | - |
Regarding overall survival, study results diverged in the first-line therapy setting. Of note, overall survival rates were assessed as secondary endpoints in the studies described below. This is consistent with standard practice, as survival rates in patients with HR-receptor-positive, HER2-negative breast cancer extend over several years, making progression-free survival the preferred primary endpoint[19]. This, in turn, affects the interpretation of overall survival data.
The MONALEESA-2 trial showed a significant overall survival benefit with ribociclib plus letrozole in the first-line therapy compared to letrozole alone[11]. The median overall survival was 63.9 months with ribociclib plus letrozole vs. 51.4 months with letrozole alone (HR 0.76; P = 0.008) after a median follow-up of 6.6 years[11]. The MONARCH-3 trial that investigated the efficacy of abemaciclib plus NSAI as first-line therapy compared to NSAI alone reported significantly longer progression-free survival and superior objective response rates with the addition of abemaciclib; final overall survival data have not yet been reported[12].
The PALOMA-2 trial that investigated the efficacy of palbociclib combined with letrozole compared to letrozole alone reported significantly longer progression-free survival with palbociclib plus letrozole (27.6 vs. 14.5 months; HR 0.563; P < 0.0001) after a median follow-up of 38 months[20].
For these reasons, the overall survival rates presented at the Annual Meeting of the American Society of Clinical Oncology in 2022 were surprising. At a median follow-up of 90 months, the median overall survival with palbociclib plus letrozole was 53.9 months compared to 51.2 months with letrozole alone (HR 0.956;
Preliminary insights from subgroup analyses with balanced amounts of missing follow-up data were presented at the American Society of Clinical Oncology Annual Meeting 2022[23]. They showed a benefit of palbociclib plus letrozole for patients with an Eastern Cooperative Oncology Group performance status of 1 or 2 (HR 0.801), disease-free interval of more than 12 months (HR 0.728), prior endocrine therapy (HR 0.801), and bone-alone disease (HR 0.712)[23]. Particularly outstanding was a median overall survival of 66.3 months for patients in the palbociclib-letrozole-arm with a disease-free interval of more than 12 months and a proportion of 10% of patients who continued to receive palbociclib plus letrozole after a median follow-up of 90 months[21]. To date, the final publication of these results is pending. Nevertheless, based on the available study results, the combination therapy of ribociclib plus endocrine therapy should be the preferred treatment regimen.
In second-line therapy, adding all three CDK4/6 inhibitors significantly prolonged progression-free and overall survival, as reported by the MONALEESA-3 trial for ribociclib, the MONARCH-2 trial for abemaciclib, and the PALOMA-3 trial for palbociclib [Table 3][15,21,24-26]. After a median follow-up of 44.8 months, the PALOMA-3 trial reported numerically longer overall survival for palbociclib plus fulvestrant compared to fulvestrant alone; however, the difference in the overall study group was not significant[24]. In patients who had sensitivity to prior endocrine therapy, the addition of palbociclib resulted in significantly longer overall survival (39.7 vs. 29.7 months; HR 0.72; CI: 0.55-0.94)[24]. After an extended follow-up of 73.3 months, the improvement in overall survival in the palbociclib-fulvestrant-arm was statistically significant in the entire study group[25].
Selected trials investigating CDK4/6 inhibitors as ≥ second-line therapy
| Trial | n | Agent | Median PFS (mo.) | HR [95% CI] | Median OS (mo.) | HR [95% CI] |
| PALOMA-3 (phase III trial)[25,136] | 521 | Palbociclib + fulvestrant vs. fulvestrant | 9.5 vs. 4.6 | 0.46 [0.36- 0.59] | 34.8 vs. 28.0 | 0.81 [0.65- 0.99] |
| MONARCH-2 (phase III trial)[26,137] | 669 | Abemaclclib + fulvestrant vs. fulvestrant | 16.4 vs. 9.3 | 0.553 [0.449-0.681] | 46.7 vs. 37.3 | 0.757 [0.606-0.945] |
| MONALEESA-3 (phase III trial)[138,139] | 726 | Ribociclib + fulvestrant vs. fulvestrant | 20.5 vs. 12.8 | 0.593 [0.480-0.732] | 53.7 vs. 41.5 | 0.73 [0.59-0.90] |
Premenopausal women are treated like postmenopausal patients after adding a gonadotropin-releasing hormone agonist like goserelin to suppress ovarian function or bilateral ovariectomy[27]. The benefit of adding ribociclib to endocrine therapy and goserelin was examined separately for premenopausal women in the MONALEESA-7 trial[10,28]. The median progression-free survival was significantly prolonged to 23.8 months with ribociclib plus endocrine therapy compared to 13.0 months with endocrine therapy alone
Side effect management is essential when using a CDK4/6 inhibitor to improve patient adherence. Table 4 briefly overviews the most common grade 3 or 4 adverse events for each CDK4/6 inhibitor. A meta-analysis showed a significant increase in the rate of grade 3 and 4 adverse events with an addition of a CDK4/6 inhibitor to endocrine therapy compared to endocrine therapy alone, including neutropenia (HR 57.05;
| Adverse event | Abemaciclib plus ET | Palbociclib plus ET | Ribociclib plus ET | |||
| Neutropenia | All grades | Grade 3/4 | All grades | Grade 3/4 | All grades | Grade 3/4 |
| Infection | 45.1% | 25.4% | 81% | 65% | 74.3% | 59.3% |
| Febrile | n.s. | n.s. | 42% | 2-3% | 50.3% | 4.2% |
| neutropenia | < 1% | n.s. | 1% | 1% | 1.5% | n.s. |
| Anemia | 30.1% | 7.1% | 28% | 3% | 18.6% | 1.2% |
| AST elevation | 14.2% | 2.9% | 7% | 3% | 15.0% | 5.7% |
| ALT elevation | 15.1% | 5.1% | 6% | 2% | 15.6% | 9.3% |
| Diarrhea | 84.6% | 11.7% | 21% | < 1% | 35.0% | 1.2% |
| Vomiting | 27.7% | 1.2% | 17% | < 1% | 29.3% | 3.6% |
| Fatigue | 40.5% | 2.3% | 39% | 2% | 36.5% | 2.4% |
Based on these studies, international guidelines recommend the addition of a CDK4/6 inhibitor to endocrine therapy in the first-line treatment of metastatic HR-positive breast cancer[27]. The combination therapy is effective for newly diagnosed or recurrent advanced breast cancer in first- or second-line therapy and in cases of primary or secondary endocrine resistance[27]. However, the resistance to CDK4/6 inhibitors is a persistent challenge and a main of research[5,30-32]. It is not fully understood whether the development of resistance is associated with overcoming cell cycle inhibition or bypassing it by activating other signaling pathways instead[5]. In general, CDK4/6 form a complex with cyclin D to phosphorylate the retinoblastoma protein, leading to the release of transcription factors (especially E2F) that activate DNA transcription
Figure 1. Mechanism of CDK4/6 inhibition; Modified from Li et al., 2020[5]. FGFR: Fibroblast growth factor receptor; ER: estrogen receptor; AI: aromatase inhibitor; CDK4/6: cyclin-dependent kinases 4/6; P: phosphorylation; Rb: retinoblastoma protein;
PROMISING APPROACHES IN THE TREATMENT OF HR-POSITIVE BREAST CANCER
Retreatment with a CDK4/6 inhibitor after disease progression on CDK4/6 inhibition
Several studies investigated the benefit of further treatment with a CDK4/6 inhibitor after progression on the first-line treatment with a CDK4/6 inhibitor. Wander et al. investigated the efficacy of abemaciclib after disease progression on a prior CDK4/6 inhibitor (primarily palbociclib) in a retrospective cohort study[38]. This study was based on several unique pharmacological properties of abemaciclib compared to palbociclib or ribociclib and the demonstration of the efficacy of abemaciclib as a single-agent treatment in heavily pretreated patients in the phase II MONARCH-1 trial[39]. Most patients received abemaciclib as non-sequential therapy with ≥ 1 intervening regimen[38]. The survival rates reported by Wanderer et al. were similar to those seen in the MONARCH-1 trial, in which patients had not previously received a CDK4/6 inhibitor, and emphasized the efficacy of abemaciclib after progression on a prior CDK4/6 inhibitor[38,39]. Furthermore, several genomic alterations previously associated with CDK4/6 resistance were detected in patients with rapid progression on abemaciclib[38].
Kalinsky et al. reported positive results from the phase II MAINTAIN trial[40]. They showed a significant benefit in progression-free survival for patients who received ribociclib in combination with a switch of endocrine therapy after progression on a CDK4/6 inhibitor plus endocrine therapy compared to the group of patients who had a switch of endocrine therapy without adding ribociclib[40]. Unfortunately, it is impossible to deduce from the study design whether changing the CDK4/6 inhibitor to ribociclib alone (without a switch of endocrine therapy) would be sufficient to show a survival benefit. Several studies are currently underway to evaluate the value of subsequent treatment with a CDK4/6 inhibitor after prior progression on a CDK4/6 inhibitor. Although further studies are needed to draw definitive conclusions, the positive signals are sufficient to conclude that a subset of patients benefits from retreatment with a CDK4/6 inhibitor.
Estrogen receptor 1 mutation
In HR-positive breast cancer, a central mechanism of acquired endocrine resistance, particularly to aromatase inhibitors, is a mutation in the drug target itself, such as a gain-of-function mutation in the ligand-binding domain of ESR1[41,42]. ESR1 mutations lead to ligand-independent estrogen receptor (ER) activity, promoting tumor growth and resistance to endocrine therapy[43]. The prevalence of ESR1 mutations depends on the prior duration of endocrine therapy and is detectable in 20%-40% of patients who have received an AI for metastatic breast cancer[42,44].
Interestingly, the mutation rate is much lower in the case of recurrent breast cancer and is less than 1% in endocrine therapy-naive patients, suggesting that ESR1 mutations are acquired mutations during AI treatment in the metastatic setting[42]. ESR1 mutations result in estrogen-independent activation of estrogen receptors and lead to resistance to AIs but not SERDs or SERMs[44-47]. ESR1 mutations usually occur with several other genomic alterations and are only partially responsible for the resistance developed[42]. Nevertheless, molecular testing can be helpful in case of cancer progression during endocrine therapy to predict resistance to AI therapy in the future.
There are several ways to detect ESR1 mutations. One is the non-invasive detection of circulating tumor DNA (ctDNA) in the patient’s plasma[48-50]. Analysis of the SoFEA trial showed that patients with ESR1 mutations had significantly better progression-free survival with fulvestrant than exemestane (HR 0.52;
There is preliminary evidence that ESR1 mutation status helps select further treatment options and monitor ongoing endocrine therapies. The PADA-1 trial was designed to demonstrate the efficacy of periodic monitoring of patients treated with palbociclib plus AI for emerging or rising ESR1 mutations in ctDNA to initiate an early treatment change to palbociclib plus fulvestrant, even before evidence of disease progression is apparent[52]. In the first step, 1017 patients were enrolled and treated with palbociclib plus an AI. After a median of 15.6 months, 172 patients with rising ESR1 mutations were randomized to continue therapy or to switch the therapy regime to palbociclib plus fulvestrant[53]. Preliminary data showed that the median progression-free survival in the palbociclib-AI-arm was 5.7 months vs. 11.9 months in the cohort that switched the treatment regime to fulvestrant plus palbociclib[53]. A preliminary analysis of safety outcomes confirmed the favorable safety profile of palbociclib in combination with any AI with or without a switch to fulvestrant[54]. Suppose these findings are confirmed in the final analysis and further studies. In that case, ESR1 mutation analysis will have clinical implications as an emerging biomarker for endocrine therapy decision-making in the future or could be used for early modification of therapy regimes to avoid expectable tumor progression even before it becomes apparent[52].
Oral selective ER degraders
Fulvestrant is a SERD that treats advanced HR-positive breast cancer; however, the application is limited to intramuscular injection. Fulvestrant antagonizes estrogen receptor alpha (ERα) and induces its degradation by binding the ligand-binding pocket[55,56]. Mutations in the ligand-binding-domain of ESR1 are responsible for resistance to AIs and reduce the potency of fulvestrant[43,57]. Efforts are currently underway to identify next-generation, orally effective SERDs with improved efficacy and potency by optimizing the molecule’s ability to saturate ERα, antagonizing its activity, and reducing its degradation[58].
Elacestrant is the first oral SERD that demonstrated better efficacy than endocrine therapy in patients with advanced HR-positive breast cancer[59]. The novel SERD functions by degrading ERα and inhibiting estradiol-dependent estrogen receptor-related gene transcription and tumor growth with improved pharmacological properties compared to fulvestrant[59-62]. The phase III EMERALD trial investigated the efficacy and safety of elacestrant in postmenopausal women with HR-positive, HER2-negative advanced or metastatic breast cancer who had progression after first- or second-line treatment with a combination of CDK4/6 inhibitor plus endocrine therapy[59]. Elacestrant was administered orally daily, and efficacy was compared with standard-of-care (SOC) endocrine therapy (fulvestrant/anastrozole/letrozole/exemestane monotherapy)[59]. Bidard et al. reported a significantly prolonged progression-free survival in patients treated with elacestrant compared to the SOC-cohort after a median follow-up of 15.1 months (HR 0.7;
The EMERALD trial reported superior progression-free survival rates of elacestrant compared to fulvestrant in this selected population and might give a first hint that elacestrant could be an orally available alternative to the intramuscular injections of fulvestrant, especially for ESR1-mutated breast cancers[59].
Several promising oral SERDs are currently being investigated in numerous clinical trials. Table 5 provides a brief overview of selected clinical trials on oral SERDs, although not all studies have reported results. Recently, the first results of the phase II acelERA trial were reported. The study evaluated the efficacy and safety of giredestrant compared with endocrine therapy (fulvestrant or an AI) in patients with HR-positive, HER2-negative advanced breast cancer who progressed after one or two lines of systemic therapy[63]. Prior treatment with fulvestrant and a CDK4/6 inhibitor was allowed. The study did not meet its primary endpoint of investigator-assessed progression-free survival; however, monotherapy with giredestrant showed a numerical improvement of progression-free survival compared with endocrine therapy of physician’s choice (5.6 months vs. 5.4 months; HR 0.81; P = 0.1757)[63]. A higher clinical benefit rate and objective response rate were reported with giredestrant[63]. In the cohort of patients with proven ESR1 mutation (39% of patients), the benefit in progression-free survival was even more pronounced (HR 0.60;
Selected trials investigating oral SERDs
| Trial | Agent | Study population | Median PFS (mo.) |
| EMERALD (phase III trial)[59] | Elacestrant vs. standard of care | Patients with ER-positive, HER2-negative advanced breast cancer who received one or two lines of endocrine therapy; required pretreatment with a cyclin-dependent kinase 4/6 inhibitor, and no more than one prior line of chemotherapy | 2.8 vs. 1.9; HR 0.70; P = 0.002 ESR1-mutant subgroup: 3.8 vs. 1.9; HR 0.55; P = 0.0005 |
| AcelERA BC (phase II trial)[63] | Giredestrant vs. endocrine treatment of physician’s choice (fulvestrant or aromatase inhibitor) | Post- and pre-/peri-menopausal women, or men with ER-positive, HER2-negative locally advanced or metastatic breast cancer who progressed after 1-2 lines of systemic therapy | 5.6 vs. 5.4 HR 0.81 P = 0.18 ESR1-mutant subgroup: 5.3 vs. 3.5; HR 0.60; P = 0.0610 |
| PersevERA (phase III trial)[141] | Giredestrant or letrozole plus palbociclib | Patients with ER-positive, HER2-negative advanced breast cancer who had no prior treatment for advanced disease | No results reported yet |
| AMEERA-5 (phase III trial)[67,68] | Amcenestrant or letrozole plus palbociclib | Patients with ER-positive, HER2-negative advanced breast cancer who have not received any prior systemic anticancer therapy for advanced disease | No results reported yet; trial discontinued based on the outcome of a prespecified interim analysis as the combination of amcenestrant plus palbociclib did not meet the prespecified boundary for continuation |
| AMEERA-3 (phase II trial)[64] | Amcenestrant vs. endocrine treatment of physician’s choice (fulvestrant in 90% of cases) | Patients with ER-positive, HER2-negative metastatic or locally advanced breast cancer who received ≤ 2 prior lines of endocrine therapy and no more than one prior line of chemotherapy or targeted therapy for advanced breast cancer. Prior treatment with cyclin-dependent kinase 4/6 inhibitor was allowed. | 3.6 vs. 3.7; HR 1.051; P = 0.6437 |
| SERENA-4 (phase III trial)[142] | Camizestrant or anastrozole plus palbociclib | Patients with ER-positive, HER2-negative advanced breast cancer who have not received any systemic treatment for advanced disease | No results reported yet |
Amcenestrant is another oral SERD that showed promising antitumor activity in phase I/II studies regardless of the ESR1 mutation status[65,66]. The efficacy of amcenestrant compared to endocrine therapy of physician’s choice was investigated in the phase II AMEERA-3 trial in postmenopausal women with HR-positive, HER2-negative advanced breast cancer who received ≤ 2 prior lines of endocrine therapy and ≤ 1 prior chemotherapy or ≤ 1 targeted therapy for advanced disease[64]. Unfortunately, the study did not meet its primary endpoint, as progression-free survival was similar in both cohorts (median progression-free survival 3.6 vs. 3.7 months; HR 1.051)[64]. Amcenestrant as monotherapy showed no clinical benefit in patients that progressed during endocrine therapy. The reasons for the differences seen between the EMERALD trial and AMEERA-3 trial could be due to differences in the patient populations studied (e.g., ESR1 mutation rates or pretreatment with CDK4/6 inhibitors). In addition, the AMEERA-5 trial investigated the efficacy and safety of amcenestrant in combination with palbociclib, was recently discontinued due to negative results of a prespecified interim analysis showing that the treatment regime failed to meet the prespecified boundary over the control arm. In contrast, no new safety signals were observed[67,68]. A publication of the results is currently pending.
PI3K/AKT/mTOR pathway in HR-positive, HER2-negative advanced breast cancer
Many mechanisms (including alterations in intracellular signaling pathways critical for cell replication and survival) lead to the development of cancer itself, metastasis, or endocrine resistance. One essential pathway that is affected by mutations in more than 70% of HR-positive breast cancer cases is the PI3K/AKT/mTOR pathway[37,69]. This pathway plays an essential role in developing endocrine resistances by stabilizing the CDK4/6 complex and reversing the effect of CDK4/6 inhibition[5,69,70]. Several interactions exist between the PI3K/AKT/mTOR pathway and the ER pathway[69]. Some of the interactions and therapeutic targets are shown in Figure 2. Targeting the PI3K/AKT/mTOR pathway is a promising approach to overcoming endocrine resistance and has been investigated by numerous studies, some of which have already shown a significant impact on survival rates[69]. Some selected studies are reviewed in more detail below.
Figure 2. Targeting the PI3K/AKT/mTOR pathway; Modified from Du Rusquec et al., 2020[69]. E: Estrogen; ER: estrogen receptor; GFR: growth factor receptor; PI3K: phosphatidylinositol-3 kinase; PI3Ki: phosphatidylinositol-3 kinase inhibitor; AKT: protein kinase B; AKTi: AKT kinase inhibitor; PTEN: phosphatase and tensin homolog; mTORC1/2: mammalian target of rapamycin complex 1/2; mTORC1/2i: mammalian target of rapamycin complex 1/2 inhibitor.
mTOR inhibition
The most clinically relevant substance targeting the PI3K/AKT/mTOR pathway is everolimus, an mTOR complex 1 (mTORC1) inhibitor. The combination of everolimus and exemestane was used in clinical practice before CDK4/6 inhibitors emerged and remaines a mainstay in treating HR-positive breast cancer[71-76]. In the phase III BOLERO-2 trial, exemestane plus everolimus was compared to exemestane plus placebo in patients with HR-positive advanced breast cancer who had recurrence or progression during prior therapy with a NSAI[71]. The addition of everolimus to exemestane resulted in a clinically significant improvement in progression-free survival in the overall population and all subgroup analyses, including patients with visceral metastases[71-73]. In terms of median overall survival, the addition of everolimus did not result in statistically significant improvement (31.0 months for everolimus plus exemestane vs. 26.6 months for placebo plus exemestane; HR 0.89; log-rank P = 0.14)[74]. The most common grade 3 and 4 adverse events were stomatitis, anemia, hyperglycemia, fatigue, and pneumonitis, which occurred more frequently with everolimus than with placebo[71].
For confirmation, Im et al. initiated the EVEREXES trial involving 235 patients, including 199 from Asia[75]. The study investigated the efficacy of everolimus in combination with exemestane in postmenopausal women with HR-positive, HER2-negative advanced breast cancer previously treated with a NSAI[75]. Median progression-free survival in the Asian subgroup was similar to the survival in the overall population
Mo et al. reported a smaller progression-free survival benefit from treatment with everolimus plus exemestane in patients previously treated with a CDK4/6 inhibitor compared to patients who were not
There have been approaches with other mTOR inhibitors. Temsirolimus is an mTOR inhibitor that is selective for mTORC1. The HORIZON trial was designed to evaluate the clinical outcome and safety of adding temsirolimus to letrozole in AI-naive patients[80]. The study included 1112 postmenopausal patients with AI-naive, HR-positive advanced breast cancer[80]. Data analysis showed no improvement in progression-free survival in the temsirolimus-letrozole group compared to adding a placebo to letrozole[80]. On the other hand, significantly more adverse events, such as hyperglycemia, diarrhea, and stomatitis, were reported with temsirolimus[80]. Based on these results, it was speculated that the lack of improvement in survival was due to cancer cells in the metastatic setting not being exposed to endocrine therapy earlier. The cancer cells might not have depended on the PI3K/mTOR pathway and were thus insensitive to mTOR inhibition[81].
Another approach was taken with sapanisertib, a selective mTOR inhibitor with dual specificity against mTORC1 and mTOR complex 2[82,83]. Preclinical studies supported the theory that dual inhibition of both protein complexes might significantly suppress cancer cell proliferation[84,85]. Based on this assumption,
PI3K inhibition
Approximately 40% of patients with HR-positive, HER2-negative breast cancer have activating mutations in the gene encoding the alpha catalytic subunit of phosphatidylinositol-4,5-bisphosphate 3-kinase (PIK3CA), resulting in hyperactivation of the alpha isoform of phosphatidylinositol 3-kinase (PI3Kα)[87,88]. Several preclinical studies have demonstrated interactions between the ER and PI3K signaling pathways, as inhibition of PI3K leads to the upregulation of ER signaling[89]. In addition, PI3K inhibition enhances ER function and dependence in HR-positive breast cancer[89,90]. First-generation PI3K inhibitors, also known as pan-PI3K inhibitors, target all four isoforms of class I PI3K. The most studied pan-PI3K inhibitors for breast cancer treatment are buparlisib and pictilisib[91,92]. Unselective inhibition of all PI3K isoforms is accompanied by a high incidence of adverse events and leads to high treatment discontinuation rates, as shown in Table 6. As a result, these pan-PI3K inhibitors were not further investigated in subsequent trials. Over time, isoform-specific PI3K inhibitors emerged and opened new possibilities. The most important of these so far is alpelisib, a PI3Kα-specific inhibitor. The SOLAR-1 trial investigated the efficacy of alpelisib in patients with HR-positive, HER2-negative advanced breast cancer who had previously received endocrine therapy[93]. The phase III trial compared the combination therapy of alpelisib plus fulvestrant with placebo plus fulvestrant. In the cohort of patients with PIK3CA-mutated cancer, a prolongation of progression-free survival of 11.0 months was reported in the alpelisib-fulvestrant group compared to 5.7 months in the control group (HR 0.65; P < 0.001)[93]. A clinically relevant treatment benefit was not observed for alpelisib-fulvestrant in the cohort without PIK3CA-mutated cancer[93]. PIK3CA mutation testing does not necessarily need to be performed on tumor tissue, as a preliminary analysis of ctDNA-based results showed similar effects[93,94]. Alpelisib-related adverse events of grade 3 or 4 included hyperglycemia, diarrhea, or maculopapular rash, and the percentage of patients that discontinued alpelisib due to adverse effects was 25% (compared to 4.2% in the placebo-fulvestrant-group)[93]. The latter demonstrates the importance of good adverse event management during alpelisib therapy. The final analysis of overall survival in the SOLAR-1 trial did not reach the prespecified boundaries for statistical significance but showed a numerical improvement of 39.3 months compared with 31.4 months when alpelisib vs. placebo was added to fulvestrant (HR 0.86; P = 0.15)[95]. To evaluate the clinical utility of alpelisib in the treatment algorithm of breast cancer, the BYLieve trial was designed to investigate the efficacy of alpelisib in patients who had experienced disease progression during or after treatment with a CDK4/6 inhibitor plus AI[96]. The results supported the clinical benefit of alpelisib following treatment with a CDK4/6 inhibitor[96,97]. This leads to the conclusion that in case of disease progression during treatment with a CDK4/6 inhibitor plus endocrine therapy, the patient should be evaluated for a PIK3CA mutation.
Selected PI3K inhibitors in HR-positive, HER2-negative breast cancer
| Trial | Agent | Isoform-specific inhibition of PI3K | Median PFS (mo.) | SAE (%) | Discontinuation rate (%) |
| BELLE-2 (phase III trial)[91] | Buparlisib or placebo plus fulvestrant | Pan-PI3K inhibition (α, β, δ, γ) | Total population: 6.9 vs. 5.0; HR 0.78; P = 0.00021 PIK3CA mutant ctDNA: 4.6 vs. 1.5; HR 0.58; P = 0.036 | 23 vs. 16 | 39 vs. 5.0 |
| FERGI (phase II trial)[92] | Pictilisib or placebo plus fulvestrant | Pan-PI3K inhibition | 6.5 vs. 5.1 HR 0.73 P = 0.268 | 16 vs. 1 | 34 vs. 15 |
| SOLAR-1 (phase III trial)[93] | Alpelisib or placebo plus fulvestrant | α-specific | 11.0 vs. 5.7; HR 0.65 P < 0.001 | 34.9 vs. 16.7 | 25.0 vs. 4.2 |
| SANDPIPER (phase III trial)[90] | Taselisib or placebo plus fulvestrant | β-isoform-sparing pan-PI3K inhibitor | 7.4 vs. 5.4; HR 0.7; P = 0.0037 | 32 vs. 8.9 | 16.8 vs. 2.3 |
Recently, another intensively studied agent was the β-isoform-sparing pan-PI3K inhibitor taselisib. The phase III SANDPIPER trial investigated the efficacy and safety of taselisib in combination with fulvestrant in women with disease progression or recurrence during or after treatment with an AI[90]. A statistically significant but small prolongation of progression-free survival was reported in patients with PIK3CA-mutant tumors in the taselisib-fulvestrant arm compared with the placebo-fulvestrant arm (7.4 months vs. 5.4 months; HR 0.70; P = 0.0037)[90]. The proportion of patients who experienced adverse events of grade ≥ 3 was 49.5% in the taselisib-fulvestrant arm vs. 16.4% in the placebo-fulvestrant arm, with diarrhea and hyperglycemia being the most frequently reported events[90]. Serious adverse events occurred in as many as 32% of patients receiving taselisib (vs. 16.4% in the placebo arm)[90]. In total, 16.8% of patients in the taselisib arm discontinued treatment, compared with 8.9% in the placebo arm[90]. Given the safety profile and modest clinical benefit, the authors concluded that taselisib had no clinical benefit despite meeting the primary endpoint[90].
In summary, the PI3K pathway is involved in many mechanisms, including carcinogenesis, proliferation, and development of resistance in HR-positive breast cancer. Currently, alpelisib is the only available agent for patients with PIK3CA mutation. Although several PI3K inhibitors have been developed and are under evaluation in various stages of clinical trials, many agents have demonstrated only modest clinical benefit, with high rates of high-grade adverse events and treatment discontinuation rates. This is partly due to the inhibition of the p110alpha subunit of PI3K, which is physiologically involved in glucose metabolism and, when inhibited, responsible for hyperglycemia[98]. Nevertheless, inhibition of the PI3K pathway remains a highly interesting research target, mainly because the clinical application of PI3K pathway inhibition is not limited to HR-positive breast cancer. A deeper understanding of the secondary effects of PI3K inhibition and management of adverse events is necessary to improve PI3K-specific treatment. Furthermore, several studies investigated the efficacy of combined inhibition of the PI3K/AKT/mTOR pathway, CDK4/6, and ER signaling pathway to overcome the acquired resistance to CDK4/6 inhibition[99,100].
Michaloglou et al. demonstrated that combining an mTOR inhibitor with a CDK4/6 inhibitor resulted in a more durable growth arrest of cancer cells and delayed the development of resistance in vitro[99]. Similarly, several studies confirmed that the triple combination of a PI3K inhibitor, CDK4/6 inhibitor, and endocrine therapy could reverse endocrine resistance in vitro[30,100]. In the future, a combination of these therapies could lead to a long-lasting therapeutic effect. Clinical trials are ongoing.
AKT inhibition
Another frequently mutated tumor-suppressor gene is phosphatase and tensin homolog (PTEN) which functions as a negative regulator of the PI3K/AKT/PTEN pathway. In the HR-positive metastatic breast cancer subgroup, approximately 5%-10% of patients harbor a somatic mutation[101,102]. The mutation leads to loss of function and is associated with a poor prognosis and resistance to endocrine therapy[103-105]. Less frequently, alteration of the PI3K/AKT pathway is caused by AKT substitution or amplification[87,106]. Because a high proportion of HR-positive breast cancers exhibit hyperactivation of the PI3K/AKT pathway, several AKT kinase inhibitors have been investigated in clinical trials. The most promising AKT kinase inhibitor to date is capivasertib. Capivasertib is an oral pan-AKT kinase inhibitor investigated in several studies and demonstrated antitumor activity[107-110]. The efficacy of capivasertib in combination with fulvestrant was tested in patients with PTEN-mutant HR-positive metastatic breast cancer in a phase I multipart expansion study by Smyth et al.[108]. The results showed a clinical benefit rate 17% in fulvestrant-naive and 42% in fulvestrant-pretreated patients after 24 weeks and an objective response rate of 8% vs. 21%[108]. Co-mutations occurred in PIK3CA, ESR1, and TP53, with a clonal dominance of PTEN in most patients[108]. In conclusion, the study reported the efficacy and antitumor activity of capivasertib plus fulvestrant with an acceptable safety profile in this heavily pretreated cohort[108]. The results showed slightly better efficacy in fulvestrant-pretreated patients, but notable phenotypic and genomic differences were observed between the cohorts[108].
The FAKTION trial is another study evaluating the AKT kinase inhibitor capivasertib. The phase II study investigated the effect of adding capivasertib to fulvestrant on progression-free survival in patients with AI-resistant advanced breast cancer[109]. Median progression-free survival was significantly longer in the capivasertib plus fulvestrant group than in the placebo-fulvestrant group (10.3 vs. 4.8 months; HR 0.58;
Pathogenic variants in DNA-repair-related genes
In addition to identifying mechanisms leading to acquired endocrine resistance, the detection of germline mutations has become a mainstay of individualized tumor therapy. It is increasingly becoming possible to perform comprehensive genomic tumor profiling using a variety of multigene assays that allow the detection of potentially treatable genetic alterations[112]. In unselected populations of breast cancer patients, approximately five percent carry a germline BReast CAncer (BRCA) gene mutation[113,114]. A mutation in the BRCA 1 gene is known to predispose to triple-negative breast cancer, while patients with BRCA 2 mutation are most likely to develop HR-positive breast cancer[115,116]. Germline testing of patients with HER2-negative metastatic breast cancer for a BRCA 1/2 mutation as a predictive biomarker for the efficacy of poly(adenosine diphosphate-ribose) polymerase (PARP) inhibition is now part of the mandatory diagnostic workup in metastatic breast cancer. PARP inhibitors were initially used in treating ovarian cancer, where PARP inhibition has become an integral part of the treatment algorithm[117-119]. Recently, PARP inhibition has also been shown to have a promising effect and clinically meaningful benefit in patients with metastatic breast cancer and germline BRCA mutation[120-123]. In the OlympiAD trial, olaparib monotherapy provided a significant benefit over standard therapy in patients with HER2-negative metastatic breast cancer who had not received more than two prior chemotherapy regimens for metastatic disease[120]. Median progression-free survival was significantly longer (7.0 months vs. 4.2 months; P < 0.001), and the response rate was significantly higher (59.9% vs. 28.8%) compared to the standard therapy group[120]. At the same time, fewer high-grade adverse events were observed with olaparib monotherapy, resulting in a lower treatment discontinuation rate due to toxic effects[120]. Meanwhile, another study also demonstrated an overall survival benefit with olaparib monotherapy in patients receiving olaparib as first-line therapy[121]. Similar positive results were reported by the EMBRACA trial, which investigated the efficacy of talazoparib, another PARP inhibitor[122]. Talazoparib provided a significant benefit over standard chemotherapy in terms of progression-free survival (8.6 months vs. 5.6 months; P < 0.001) and patient-reported outcomes[122].
One of the selection criteria of the described studies was a proven germline mutation. However, monotherapy with olaparib also showed positive effects in the presence of a somatic BRCA mutation in a phase II trial by Tung et al.[124]. The same study investigated the effect of olaparib in patients with metastatic breast cancer and mutations in homologous recombination-related genes other than BRCA 1/2. However, it confirmed an improvement in progression-free survival only in patients with germline “partner and localizer of BRCA 2” (PALB2) mutation[124]. These results may indicate that the population of breast cancer patients who could benefit from PARP inhibition could be expanded through further studies.
A recently published, retrospective real-world study by Bruno et al. investigated the impact of an existing germline pathogenic variant in a DNA repair-related gene on the therapeutic efficacy of a CDK4/6 inhibitor[125]. Reported pathogenic variants were germline mutations in the BRCA 1/2, Ataxia Telangiectasia Mutated, and Checkpoint kinase 2 genes[125]. A proven germline mutation was associated with a shorter median progression-free survival (10.2 months) compared with patients without these mutations
Histone deacetylase inhibition - an example of an epigenetic approach
Unlike genetic mutations, epigenetic alterations are not due to mutations in the primary DNA sequence but cause changes in gene expression. The role of epigenetics in tumor progression and the development of endocrine resistance in HR-positive breast cancer is an emerging field of clinical investigation[126]. There are first promising approaches to reverse to effects of epigenetic alterations by epigenetic modifiers, such as histone deacetylase (HDAC) inhibitors[127]. Entinostat is an orally administered HDAC inhibitor currently under investigation as it has shown potential antiproliferative activity in breast cancer cells[128]. On the pharmacological level, it functions by inhibiting the enzyme histone deacetylase, which is thought to play an essential role in regulating gene expression through epigenetic modifications[127]. HDAC inhibition leads to the downregulation of estrogen-independent signaling pathways and causes a normalization of ER levels[129,130]. Available data show controversial results regarding the impact of entinostat on survival rates[127,131,132]. A phase II study investigated the influence of the addition of entinostat vs. placebo once a week to the daily application of exemestane in postmenopausal women with HR-positive advanced breast cancer who had tumor progression on a NSAI[133]. The addition of entinostat improved median progression-free survival to 4.3 months vs. 2.3 months with placebo (HR 0.73; one-sided P = 0.055; two-sided P = 0.11) and median overall survival to 28.1 months vs. 19.8 months with placebo (HR 0.59; P = 0.036)[133]. Entinostat was generally well tolerated, with leading adverse events of grade 3 or 4 being fatigue and neutropenia[133]. A phase III trial with a Chinese patient cohort with advanced HR-positive breast cancer also reported a positive impact of entinostat[132]. Analysis of 354 enrolled patients showed an improved progression-free survival of 6.32 months in the exemestane-entinostat-arm compared to 3.72 months in the exemestane-placebo-arm (HR 0.74; P < 0.001)[132]. Reported adverse events included neutropenia, thrombocytopenia, and leucopenia, all of them significantly more often in patients treated with entinostat[132]. In contrast, another randomized phase III trial with similar inclusion criteria reported no improvement in progression-free (3.3 vs. 3.1 months; HR 0.87; P = 0.03) or overall survival (23.4 vs. 21.7 months; HR 0.99; P = 0.94), although the pharmacodynamic analysis confirmed the target inhibition in the entinostat-treated cohort[131]. Nevertheless, epigenetic modifiers open a new field of research in treating HR-positive breast cancer that will certainly gain importance in the future. Furthermore, preclinical data provide evidence that combining entinostat with palbociclib enhances the antitumoral activity of both drugs[134]. Further clinical studies on this novel and promising substance are still pending.
CONCLUSION
Endocrine therapeutic strategies are currently being improved and expanded by adding molecularly-targeted substances. These play an increasingly important role in managing advanced hormone receptor-positive breast cancer, particularly given the central problem of resistance to endocrine therapy. The search for biomarkers must be intensively pursued to individualize cancer therapies further. CDK4/6 inhibitors, in addition to endocrine therapy, have already become the standard of care in the first-line treatment of HR-positive advanced breast cancer. However, several novel promising substances (e.g., oral SERDs or PI3K inhibitors) are also being investigated in clinical trials, so an expansion of therapy options can be expected shortly. Given the rapid scientific progress, knowledge and management of adverse events are critical, as the new targeted agents are associated with side effects that differ significantly from endocrine therapy alone.
DECLARATIONS
Authors’ contributionsMade substantial contributions to conception and design of the study and performed data analysis and interpretation: Droste A, Schmidt M
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestMarcus Schmidt has received personal fees from AstraZeneca, BioNTech, Daiichi Sankyo, Eisai, Lilly, MSD, Novartis, Pantarhei Bioscience, Pfizer, Pierre Fabre, Roche, and SeaGen. His institution has received research funding from AstraZeneca, BioNTech, Eisai, Genentech, German Breast Group, Novartis, Palleos, Pantarhei Bioscience, Pierre Fabre, and SeaGen. In addition, he has a patent for EP 2390370 B1 and a patent for EP 2951317 B1 issued. Annika Droste reports no conflict of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 2017;3:524-48.
2. Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001;98:10869-74.
3. Buzdar AU. Endocrine therapy in the treatment of metastatic breast cancer. Semin Oncol 2001;28:291-304.
4. O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol 2016;13:417-30.
5. Li Z, Zou W, Zhang J, et al. Mechanisms of CDK4/6 inhibitor resistance in luminal breast cancer. Front Pharmacol 2020;11:580251.
6. Martin M, Zielinski C, Ruiz-Borrego M, et al. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: a phase III randomised controlled trial-PEARL. Ann Oncol 2021;32:488-99.
7. Park YH, Kim TY, Kim GM, et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol 2019;20:1750-9.
8. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015;16:25-35.
9. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016;375:1925-36.
10. Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 2018;19:904-15.
11. Hortobagyi GN, Stemmer SM, Burris HA, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med 2022;386:942-50.
12. Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019;5:5.
13. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016;375:1738-48.
14. Im SA, Lu YS, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 2019;381:307-16.
15. Slamon DJ, Neven P, Chia S, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med 2020;382:514-24.
16. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017;35:3638-46.
17. Li J, Huo X, Zhao F, et al. Association of cyclin-dependent kinases 4 and 6 inhibitors with survival in patients with hormone receptor-positive metastatic breast cancer: a systematic review and meta-analysis. JAMA Netw Open 2020;3:e2020312.
18. Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol 2018;29:1541-7.
19. Seidman AD, Bordeleau L, Fehrenbacher L, et al. National cancer institute breast cancer steering committee working group report on meaningful and appropriate end points for clinical trials in metastatic breast cancer. J Clin Oncol 2018;36:3259-68.
20. Rugo HS, Finn RS, Diéras V, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat 2019;174:719-29.
21. Finn RS, Rugo HS, Dieras VC, et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer (ER+/HER2- ABC): analyses from PALOMA-2. JCO 2022;40:LBA1003.
22. Pfizer. Pfizer announces overall survival results from phase 3 PALOMA-2 trial of IBRANCE® (palbociclib) for the first-line treatment of ER+, HER2- metastatic breast cancer. Available from: www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-overall-survival-results-phase-3-paloma-2 [Last accessed on 16 Feb 2023].
23. Helwick, caroline. paloma-2: no overall survival benefit reported with palbociclib/letrozole in Advanced breast cancer. The ASCO post. Available from: https://ascopost.com/issues/august-10-2022/no-overall-survival-benefit-reported-with-palbociclibletrozole-in-advanced-breast-cancer/ [Last accessed on 16 Feb 2023].
24. Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 2018;379:1926-36.
25. Cristofanilli M, Rugo HS, Im S, et al. Overall survival (OS) with palbociclib (PAL) + fulvestrant (FUL) in women with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (ABC): updated analyses from PALOMA-3. JCO 2021;39:1000.
26. Sledge GW Jr, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol 2020;6:116-24.
27. Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020;31:1623-49.
28. Lu YS, Im SA, Colleoni M, et al. Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER2- advanced breast cancer in MONALEESA-7: a phase III randomized clinical trial. Clin Cancer Res 2022;28:851-9.
29. Neven P, Rugo HS, Tolaney SM, et al. Abemaciclib plus fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in premenopausal women: subgroup analysis from the MONARCH 2 trial. Breast Cancer Res 2021;23:87.
30. Herrera-Abreu MT, Palafox M, Asghar U, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res 2016;76:2301-13.
31. Pandey K, An HJ, Kim SK, et al. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: a review. Int J Cancer 2019;145:1179-88.
32. Portman N, Alexandrou S, Carson E, Wang S, Lim E, Caldon CE. Overcoming CDK4/6 inhibitor resistance in ER-positive breast cancer. Endocr Relat Cancer 2019;26:R15-30.
34. Yu Q, Sicinska E, Geng Y, et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell 2006;9:23-32.
35. Choi YJ, Li X, Hydbring P, et al. The requirement for cyclin D function in tumor maintenance. Cancer Cell 2012;22:438-51.
36. Yang C, Li Z, Bhatt T, et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene 2017;36:2255-64.
37. Miller TW, Rexer BN, Garrett JT, Arteaga CL. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res 2011;13:224.
38. Wander SA, Han HS, Zangardi ML, et al. Clinical outcomes with abemaciclib after prior CDK4/6 inhibitor progression in breast cancer: a multicenter experience. J Natl Compr Cancer Netw 2021:1-8.
39. Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2- metastatic breast cancer. Clin Cancer Res 2017;23:5218-24.
40. Kalinsky K, Accordino MK, Chiuzan C, et al. A randomized, phase II trial of fulvestrant or exemestane with or without ribociclib after progression on anti-estrogen therapy plus cyclin-dependent kinase 4/6 inhibition (CDK 4/6i) in patients (pts) with unresectable or hormone receptor-positive (HR+), HER2-negative metastatic breast cancer (MBC): MAINTAIN trial. JCO 2022;40:LBA1004.
41. Hermida-Prado F, Jeselsohn R. The ESR1 mutations: from bedside to bench to bedside. Cancer Res 2021;81:537-8.
42. Brett JO, Spring LM, Bardia A, Wander SA. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res 2021;23:85.
43. Jeselsohn R, Buchwalter G, De Angelis C, Brown M, Schiff R. ESR1 mutations-a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol 2015;12:573-83.
44. Jeselsohn R, Yelensky R, Buchwalter G, et al. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res 2014;20:1757-67.
45. Turner NC, Swift C, Kilburn L, et al. ESR1 mutations and overall survival on fulvestrant versus exemestane in advanced hormone receptor-positive breast cancer: a combined analysis of the phase III SoFEA and EFECT trials. Clin Cancer Res 2020;26:5172-7.
46. Fribbens C, O’Leary B, Kilburn L, et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol 2016;34:2961-8.
47. Toy W, Shen Y, Won H, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet 2013;45:1439-45.
48. Schiavon G, Hrebien S, Garcia-Murillas I, et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med 2015;7:313ra182.
49. Forshew T, Murtaza M, Parkinson C, et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci Transl Med 2012;4:136ra68.
50. Dawson SJ, Tsui DW, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med 2013;368:1199-209.
51. Turner NC, Ro J, André F, et al. Palbociclib in Hormone-receptor-positive advanced breast cancer. N Engl J Med 2015;373:209-19.
52. Berger F, Marce M, Delaloge S, et al. Randomised, open-label, multicentric phase III trial to evaluate the safety and efficacy of palbociclib in combination with endocrine therapy, guided by ESR1 mutation monitoring in oestrogen receptor-positive, HER2-negative metastatic breast cancer patients: study design of PADA-1. BMJ Open 2022;12:e055821.
53. Bidard F, Hardy-bessard A, Bachelot T, et al. Abstract GS3-05: fulvestrant-palbociclib vs continuing aromatase inhibitor-palbociclib upon detection of circulating ESR1 mutation in HR+ HER2- metastatic breast cancer patients: results of PADA-1, a UCBG-GINECO randomized phase 3 trial. Cancer Res 2022;82:GS3-05.
54. Delaloge S, Hardy-Bessard AC, Bachelot T, et al. First line aromatase inhibitor (AI) + palbociclib with randomized switch to fulvestrant + palbociclib upon detection of circulating ESR1 mutation in HR+ HER2-metastatic breast cancer patients: Global safety results of PADA-1, a UCBG-GINECO phase III trial. Cancer Res 2022;82:P1-18-16.
56. Pike AC, Brzozowski AM, Walton J, et al. Structural insights into the mode of action of a pure antiestrogen. Structure 2001;9:145-53.
57. Toy W, Weir H, Razavi P, et al. Activating ESR1 mutations differentially affect the efficacy of ER antagonists. Cancer Discov 2017;7:277-87.
58. Fanning SW, Greene GL. Next-generation ERα inhibitors for endocrine-resistant ER+ breast cancer. Endocrinology 2019;160:759-69.
59. Bidard FC, Kaklamani VG, Neven P, et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD trial. J Clin Oncol 2022;40:3246-56.
60. Bihani T, Patel HK, Arlt H, et al. Elacestrant (RAD1901), a selective estrogen receptor degrader (SERD), has antitumor activity in multiple ER+ breast cancer patient-derived xenograft models. Clin Cancer Res 2017;23:4793-804.
61. Wardell SE, Nelson ER, Chao CA, Alley HM, McDonnell DP. Evaluation of the pharmacological activities of RAD1901, a selective estrogen receptor degrader. Endocr Relat Cancer 2015;22:713-24.
62. Garner F, Shomali M, Paquin D, Lyttle CR, Hattersley G. RAD1901: a novel, orally bioavailable selective estrogen receptor degrader that demonstrates antitumor activity in breast cancer xenograft models. Anticancer Drugs 2015;26:948-56.
63. Martin Jimenez M, Lim E, Chavez Mac Gregor M, et al. 211MO giredestrant (GDC-9545) vs physician choice of endocrine monotherapy (PCET) in patients (pts) with ER+, HER2- locally advanced/metastatic breast cancer (LA/mBC): primary analysis of the phase II, randomised, open-label acelERA BC study. Ann Oncol 2022;33:S633-4.
64. Tolaney S, Chan A, Petrakova K, et al. 212MO AMEERA-3, a phase II study of amcenestrant (AMC) versus endocrine treatment of physician’s choice (TPC) in patients (pts) with endocrine-resistant ER+/HER2- advanced breast cancer (aBC). Ann Oncol 2022;33:S634-5.
65. Linden HM, Campone M, Bardia A, et al. Abstract PD8-08: a phase 1/2 study of SAR439859, an oral selective estrogen receptor (ER) degrader (SERD), as monotherapy and in combination with other anti-cancer therapies in postmenopausal women with ER-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer (mBC): AMEERA-1. Cancer Res 2021;81:PD8-08.
66. Bardia A, Linden HM, Ulaner GA, et al. Dose-escalation study of SAR439859, an oral selective estrogen receptor (ER) degrader (SERD), in postmenopausal women with ER+/HER2- metastatic breast cancer (mBC). JCO 2019;37:1054.
67. Bardia A, Cortes J, Hurvitz SA, et al. AMEERA-5: a randomized, double-blind phase 3 study of amcenestrant plus palbociclib versus letrozole plus palbociclib for previously untreated ER+/HER2- advanced breast cancer. Ther Adv Med Oncol 2022;14:17588359221083956.
68. Sanofi provides update on amcenestrant clinical development program - sanofi. Available from: https://www.sanofi.com/en/media-room/press-releases/2022/2022-08-17-05-30-00-2499668 [Last accessed on 16 Feb 2023].
69. Rusquec P, Blonz C, Frenel JS, Campone M. Targeting the PI3K/Akt/mTOR pathway in estrogen-receptor positive HER2 negative advanced breast cancer. Ther Adv Med Oncol 2020;12:1758835920940939.
70. Thangavel C, Dean JL, Ertel A, et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer 2011;18:333-45.
71. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012;366:520-9.
72. Beck JT, Hortobagyi GN, Campone M, et al. Everolimus plus exemestane as first-line therapy in HR+, HER2- advanced breast cancer in BOLERO-2. Breast Cancer Res Treat 2014;143:459-67.
73. Yardley DA, Noguchi S, Pritchard KI, et al. Everolimus plus exemestane in postmenopausal patients with HR+ breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther 2013;30:870-84.
74. Piccart M, Hortobagyi GN, Campone M, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2. Ann Oncol 2014;25:2357-62.
75. Im YH, Karabulut B, Lee KS, et al. Safety and efficacy of everolimus (EVE) plus exemestane (EXE) in postmenopausal women with locally advanced or metastatic breast cancer: final results from EVEREXES. Breast Cancer Res Treat 2021;188:77-89.
76. Lousberg L, Jerusalem G. Safety, efficacy and patient acceptability of everolimus in the treatment of breast cancer. Breast Cancer 2016;10:239-52.
77. Mo H, Renna CE, Moore HCF, et al. Real-world outcomes of everolimus and exemestane for the treatment of metastatic hormone receptor-positive breast cancer in patients previously treated with CDK4/6 inhibitors. Clin Breast Cancer 2022;22:143-8.
78. Cook MM, Al Rabadi L, Kaempf AJ, Saraceni MM, Savin MA, Mitri ZI. Everolimus plus exemestane treatment in patients with metastatic hormone receptor-positive breast cancer previously treated with CDK4/6 inhibitor therapy. Oncologist 2021;26:101-6.
79. Jeong H, Jeong JH, Kim JE, Ahn JH, Jung KH, Kim SB. Comparison of the effectiveness and clinical outcome of everolimus followed by CDK4/6 inhibitors with the opposite treatment sequence in hormone receptor-positive, HER2-negative metastatic breast cancer. Cancer Res Treat 2022;54:469-77.
80. Wolff AC, Lazar AA, Bondarenko I, et al. Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol 2013;31:195-202.
81. Vinayak S, Carlson RW. mTOR inhibitors in the treatment of breast cancer. Oncology 2013;27:38-44.
82. Gökmen-Polar Y, Liu Y, Toroni RA, et al. Investigational drug MLN0128, a novel TORC1/2 inhibitor, demonstrates potent oral antitumor activity in human breast cancer xenograft models. Breast Cancer Res Treat 2012;136:673-82.
83. Zeng Z, Wang RY, Qiu YH, et al. MLN0128, a novel mTOR kinase inhibitor, disrupts survival signaling and triggers apoptosis in AML and AML stem/ progenitor cells. Oncotarget 2016;7:55083-97.
84. Zhang X, Wang X, Xu T, Zhong S, Shen Z. Targeting of mTORC2 may have advantages over selective targeting of mTORC1 in the treatment of malignant pheochromocytoma. Tumour Biol 2015;36:5273-81.
85. Zou Z, Chen J, Yang J, Bai X. Targeted inhibition of rictor/mTORC2 in cancer treatment: a new era after rapamycin. Curr Cancer Drug Targets 2016;16:288-304.
86. García-Sáenz JÁ, Martínez-Jáñez N, Cubedo R, et al. Sapanisertib plus fulvestrant in postmenopausal women with estrogen receptor-positive/HER2-negative advanced breast cancer after progression on aromatase inhibitor. Clin Cancer Res 2022;28:1107-16.
87. Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012;490:61-70.
88. Goncalves MD, Hopkins BD, Cantley LC. Phosphatidylinositol 3-kinase, growth disorders, and cancer. N Engl J Med 2018;379:2052-62.
89. Bosch A, Li Z, Bergamaschi A, et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med 2015;7:283ra51.
90. Dent S, Cortés J, Im YH, et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: the SANDPIPER trial. Ann Oncol 2021;32:197-207.
91. Baselga J, Im SA, Iwata H, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:904-16.
92. Krop IE, Mayer IA, Ganju V, et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2016;17:811-21.
93. André F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med 2019;380:1929-40.
94. Juric D, Ciruelos E, Rubovszky G, et al. Abstract GS3-08: alpelisib + fulvestrant for advanced breast cancer: subgroup analyses from the phase III SOLAR-1 trial. Cancer Res 2019;79:GS3-08.
95. André F, Ciruelos EM, Juric D, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol 2021;32:208-17.
96. Rugo HS, Lerebours F, Ciruelos E, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol 2021;22:489-98.
97. Turner S, Chia S, Kanakamedala H, et al. Effectiveness of alpelisib + fulvestrant compared with real-world standard treatment among patients with HR+, HER2-, PIK3CA-mutated breast cancer. Oncologist 2021;26:e1133-42.
98. Hopkins BD, Pauli C, Du X, et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 2018;560:499-503.
99. Michaloglou C, Crafter C, Siersbaek R, et al. Combined inhibition of mTOR and CDK4/6 is required for optimal blockade of E2F function and long-term growth inhibition in estrogen receptor-positive breast cancer. Mol Cancer Ther 2018;17:908-20.
100. Vora SR, Juric D, Kim N, et al. CDK4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell 2014;26:136-49.
102. Bertucci F, Ng CKY, Patsouris A, et al. Genomic characterization of metastatic breast cancers. Nature 2019;569:560-4.
103. Carbognin L, Miglietta F, Paris I, Dieci MV. Prognostic and predictive implications of PTEN in breast cancer: unfulfilled promises but intriguing perspectives. Cancers 2019;11:1401.
104. Costa C, Wang Y, Ly A, et al. PTEN loss mediates clinical cross-resistance to CDK4/6 and PI3Kα inhibitors in breast cancer. Cancer Discov 2020;10:72-85.
105. Juric D, Castel P, Griffith M, et al. Convergent loss of PTEN leads to clinical resistance to a PI3Kα inhibitor. Nature 2015;518:240-4.
106. Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res 2008;68:6084-91.
107. Kalinsky K, Hong F, McCourt CK, et al. Effect of capivasertib in patients with an AKT1 E17K-mutated tumor: NCI-MATCH subprotocol EAY131-Y nonrandomized trial. JAMA Oncol 2021;7:271-8.
108. Smyth LM, Batist G, Meric-Bernstam F, et al. Selective AKT kinase inhibitor capivasertib in combination with fulvestrant in PTEN-mutant ER-positive metastatic breast cancer. NPJ Breast Cancer 2021;7:44.
109. Jones RH, Casbard A, Carucci M, et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2020;21:345-57.
110. Smyth LM, Tamura K, Oliveira M, et al. Capivasertib, an AKT kinase inhibitor, as monotherapy or in combination with fulvestrant in patients with AKT1 (E17K)-mutant, ER-positive metastatic breast cancer. Clin Cancer Res 2020;26:3947-57.
111. Howell SJ, Casbard A, Carucci M, et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive, HER2-negative breast cancer (FAKTION): overall survival, updated progression-free survival, and expanded biomarker analysis from a randomised, phase 2 trial. Lancet Oncol 2022;23:851-64.
112. Razavi P, Chang MT, Xu G, et al. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 2018;34:427-438.e6.
113. Malone KE, Daling JR, Doody DR, et al. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res 2006;66:8297-308.
114. Kurian AW, Gong GD, John EM, et al. Performance of prediction models for BRCA mutation carriage in three racial/ethnic groups: findings from the Northern California Breast Cancer Family Registry. Cancer Epidemiol Biomarkers Prev 2009;18:1084-91.
115. Antoniou AC, Kuchenbaecker KB, Soucy P, et al. Common variants at 12p11, 12q24, 9p21, 9q31.2 and in ZNF365 are associated with breast cancer risk for BRCA1 and/or BRCA2 mutation carriers. Breast Cancer Res 2012;14:R33.
116. Mavaddat N, Barrowdale D, Andrulis IL, et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev 2012;21:134-47.
117. Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 2019;381:2416-28.
118. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2018;379:2495-505.
119. Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1274-84.
120. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 2017;377:523-33.
121. Robson ME, Tung N, Conte P, et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol 2019;30:558-66.
122. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 2018;379:753-63.
123. Lee KH, Sohn J, Goodwin A, et al. Talazoparib versus chemotherapy in patients with HER2-negative advanced breast cancer and a germline BRCA1/2 mutation enrolled in Asian countries: exploratory subgroup analysis of the phase III EMBRACA trial. Cancer Res Treat 2021;53:1084-95.
124. Tung NM, Robson ME, Ventz S, et al. TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol 2020;38:4274-82.
125. Bruno L, Ostinelli A, Waisberg F, et al. Cyclin-dependent kinase 4/6 inhibitor outcomes in patients with advanced breast cancer carrying germline pathogenic variants in DNA repair-related genes. JCO Precis Oncol 2022;6:e2100140.
126. Connolly R, Stearns V. Epigenetics as a therapeutic target in breast cancer. J Mammary Gland Biol Neoplasia 2012;17:191-204.
127. Trapani D, Esposito A, Criscitiello C, et al. Entinostat for the treatment of breast cancer. Expert Opin Investig Drugs 2017;26:965-71.
128. Saito A, Yamashita T, Mariko Y, et al. A synthetic inhibitor of histone deacetylase, MS-27-275, with marked in vivo antitumor activity against human tumors. Proc Natl Acad Sci USA 1999;96:4592-7.
129. Connolly RM, Rudek MA, Piekarz R. Entinostat: a promising treatment option for patients with advanced breast cancer. Future Oncol 2017;13:1137-48.
130. Shah P, Gau Y, Sabnis G. Histone deacetylase inhibitor entinostat reverses epithelial to mesenchymal transition of breast cancer cells by reversing the repression of E-cadherin. Breast Cancer Res Treat 2014;143:99-111.
131. Connolly RM, Zhao F, Miller KD, et al. E2112: randomized phase III trial of endocrine therapy plus entinostat or placebo in hormone receptor-positive advanced breast cancer. a trial of the ECOG-ACRIN cancer research group. J Clin Oncol 2021;39:3171-81.
132. Xu B, Zhang Q, Hu X, et al. Abstract GS1-06: a randomized control phase III trial of entinostat, a once weekly, class I selective histone deacetylase inhibitor, in combination with exemestane in patients with hormone receptor positive advanced breast cancer. Cancer Res 2022;82:GS1-06.
133. Yardley DA, Ismail-Khan RR, Melichar B, et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol 2013;31:2128-35.
134. Lee J, Lim B, Pearson T, Tripathy D, Ordentlich P, Ueno N. Abstract P5-21-15: the synergistic antitumor activity of entinostat (MS-275) in combination with palbociclib (PD 0332991) in estrogen receptor-positive and triple-negative breast cancer. Cancer Res 2018;78:P5-21.
135. Finn RS, Boer K, Bondarenko I, et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2- advanced breast cancer (PALOMA-1, TRIO-18). Breast Cancer Res Treat 2020;183:419-28.
136. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425-39.
137. Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017;35:2875-84.
138. Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol 2018;36:2465-72.
139. Slamon DJ, Neven P, Chia S, et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol 2021;32:1015-24.
140. Rugo HS, Huober J, García-Sáenz JA, et al. Management of abemaciclib-associated adverse events in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: safety analysis of MONARCH 2 and MONARCH 3. Oncologist 2021;26:e53-65.
141. Turner NC, Jhaveri KL, Bardia A, et al. persevERA breast cancer (BC): phase III study evaluating the efficacy and safety of giredestrant (GDC-9545) + palbociclib versus letrozole + palbociclib in patients (pts) with estrogen-receptor-positive, HER2-negative locally advanced or metastatic BC (ER+/HER2- LA/mBC). JCO 2021;39:TPS1103.
142. Im S, Hamilton EP, Llombart Cussac A, et al. SERENA-4: A phase 3 comparison of AZD9833 (camizestrant) plus palbociclib, versus anastrozole plus palbociclib, for patients with ER-positive, HER2-negative advanced breast cancer who have not previously received systemic treatment for advanced disease. JCO 2021;39:TPS1101.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Droste A, Schmidt M. Endocrine therapy in metastatic breast cancer-more than just CDK4/6 inhibitors. J Cancer Metastasis Treat 2023;9:2. http://dx.doi.org/10.20517/2394-4722.2022.94
AMA Style
Droste A, Schmidt M. Endocrine therapy in metastatic breast cancer-more than just CDK4/6 inhibitors. Journal of Cancer Metastasis and Treatment. 2023; 9(1): 2. http://dx.doi.org/10.20517/2394-4722.2022.94
Chicago/Turabian Style
Droste, Annika, Marcus Schmidt. 2023. "Endocrine therapy in metastatic breast cancer-more than just CDK4/6 inhibitors" Journal of Cancer Metastasis and Treatment. 9, no.1: 2. http://dx.doi.org/10.20517/2394-4722.2022.94
ACS Style
Droste, A.; Schmidt M. Endocrine therapy in metastatic breast cancer-more than just CDK4/6 inhibitors. J. Cancer. Metastasis. Treat. 2023, 9, 2. http://dx.doi.org/10.20517/2394-4722.2022.94
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 9 clicks
Cite This Article 9 clicks



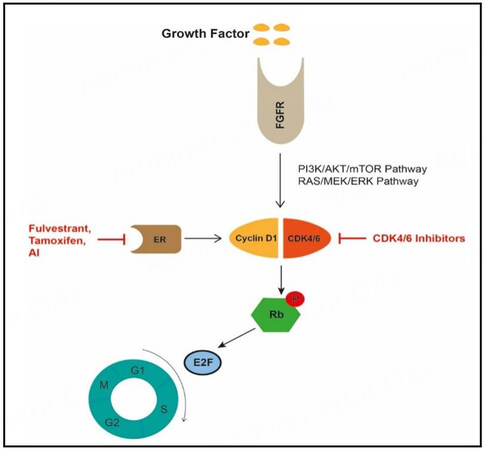
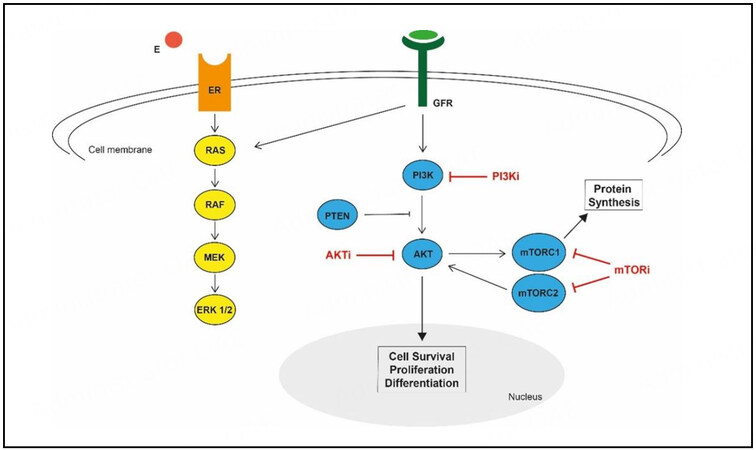








Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.