The role of pro-inflammatory components, carcinoma-associated fibroblasts, and tumor-associated macrophages in ovarian cancer progression and metastasis
Abstract
Ovarian cancer (OC) is associated with poor outcomes and challenges scientists and clinicians. It is usually diagnosed in advanced stages when it is frequently aggressive, chemoresistant, and metastatic. The most prevalent form of OC is epithelial ovarian cancer (EOC), which displays significant heterogeneity, enhancing the difficulty in managing the disease. Several factors have been associated with the disease’s development and progression, especially those related to the tumor microenvironment (TME). Here, we highlight components of the ovarian TME in the disease development process, including pro-inflammatory pathways activated by interleukins, cytokines and chemokines, cancer-associated fibroblasts, tumor-associated macrophages, and epithelial-mesenchymal transition. We compiled evidence identifying TME factors promoting the development, chemoresistance, and metastasis, including cytokines, chemokines, growth factors, and tumor-associated cells. We identify potential targets for treatment and improving outcomes. These targets block or alter pathways associated with OC (especially EOC) progression.
Keywords
INTRODUCTION
Epidemiological data on ovarian cancer (OC) reveals that it ranks fifth among cancer deaths in women[1,2] and is the most common cause of gynecologically-related cancer deaths[1,3]. The five-year survival rate is below 45%[4]. The poor outcomes are due, in part, to the absence of early symptoms, the lack of tests for early stages, and the absence of effective treatments[3,5]. Consequently, 70% of tumors are metastatic in the advanced stages (III or IV) but not in the initial phases (I or II) when the survival rates are 90%[3,5].
There were an estimated 19,880 new OC cases in the US in 2022, and about 12,810 women likely died[1]. OC primarily affects older women, with almost half diagnosed at 63 years old or older[1,6].
Epithelial ovarian cancer (EOC) accounts for 90% of OCs[7]. EOC can be classified according to morphological categories based on cellular, immunologic, and molecular profiles. These include high-grade serous ovarian carcinoma (HGSOC), low-grade serous ovarian carcinoma, mucinous ovarian carcinoma (MOC), endometrioid carcinoma (EC), and clear-cell carcinoma (CCC)[8]. EOC are subclassified according to heterogeneous molecular, cellular, and spatial pathology[7,9] as types I and II tumors[10]. Type I tumors are not as lethal as type II. They present a relatively normal karyotype, lower grade, higher frequency of mutations in the Ras signaling pathway, and (usually) no p53 or BRCA mutations. By contrast, type II EOC are typically invasive high-grade tumors associated with fatal outcomes. They comprise BRCA dysfunctions and mutations in p53, in addition to changes in DNA copy number[3,11]. Unfortunately, HGSOC accounts for 75% of all diagnosed EOC[9,12]. The literature suggests that most serous ovarian carcinomas (SOCs) originate from the fallopian tube epithelium, while endometriosis is likely the origin of EC and CCC[13,14]. MOC's origin is not fully understood; however, recent evidence suggests it arises from benign and borderline precursors in the ovary[15].
Despite its heterogeneity, all OC management involves cytoreductive surgery followed by chemotherapy combining platinum derivatives (e.g., cisplatin or carboplatin) and taxane derivatives (e.g., paclitaxel or docetaxel)[16,17]. Despite initial satisfactory responses of the disease to this treatment, most OCs relapse as an aggressive and chemoresistant disease[13,16]. These findings drive the discovery of unknown cellular and molecular aspects of OC development and progression.
In recent years, inflammation has emerged as a critical pathway in acquiring chemoresistant phenotype by cancer cells, including OC[18]. The acquisition of chemoresistant phenotype by OC cells and metastasis occurrence is associated with an intense inflammatory process that accompanies disease progression[18,19-21], as it modulates the tumor microenvironment (TME)[18].
TME is the niche where primary or metastatic tumors interact with stromal, immune, and endothelial cells, including fibroblasts and their metabolites[22]. The OC TME is exceptionally complex, comprising cells from the ovary, ascites fluid cells, omentum, and peritoneum[23]. In recent years, it has been shown that TME components are of great importance in carcinogenesis and chemoresistance, in addition to presenting a fundamental role in metastasis[24-27]. In this context, some TME cells, especially tumor-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs), and tumor-associated neutrophils, play pivotal roles in OC progression[28-30], especially in HGSOC[29,30]. Because of the role of various components of TME associated with OCs progression and metastasis, the current review collected the most current knowledge in the field to address alternatives for OC treatment.
PRO-INFLAMMATORY COMPONENTS AS TME REGULATORS
Cytokines are pro-inflammatory or anti-inflammatory components that modulate the immune system response. Thus, they are essential for the positive and negative stimulation of normal inflammatory processes[31]. In cancer, this strategy stimulates immune cells that positively ensure tumor protection or stimulate anti-tumor immunity[32,33]. Hence, inflammation and its mediators (e.g., interleukins) are directly involved in EOC development, metastasis, and chemoresistance[21,34].
Compared to normal ovarian tissues, OC secretes large amounts of interleukin-6 (IL-6), and high levels of this cytokine have been linked to OC growth, proliferation[35,36], and chemoresistance[37]. In a post-neoadjuvant chemotherapy sample, IL-6 was highly overexpressed and correlated with recurrence time, suggesting it may participate in immune TME modification[38]. Furthermore, IL-6 promotes survival and metastasis, as it regulates macrophage infiltration to ovarian tumors, supporting its role in maintaining a favorable TME for cancer progression[39,40]. Another essential protein, interleukin-34 (IL-34), modulates OC progression by promoting macrophage colony formation, which directly alters innate immunity and inflammation[41]. IL-34 is associated with growth, proliferation, and metastasis and may participate in chemoresistance because it is overexpressed following chemotherapy exposure[42]. Serum IL-6 and interleukins 8 (IL-8) and 10 (IL-10) were identified in advanced OC patients at significantly higher levels than in early-stage OC patients. This finding suggests a critical role of these cytokines in malignancy progression[43]. IL-8 has also been associated with autophagy downregulation and migration upregulation of OC cell lines[44]. In addition to immune system cell stimulation, cytokines act as guides to specific regions. Thus, they are called inflammatory chemokines[45].
Inflammatory chemokines (CC) are protein ligands that have a chemotactic role in inflammation[46,47]. In addition to attraction and repellent properties, chemokines regulate communication among immune system cells[47,48]. Many CC subfamilies of chemokine ligands (CCL) and motif chemokine ligands (CXCL) were found at high levels in various OC tissues and cells[49]. As suggested by Hornburg et al., CXCL pathways (CXCL16-CXCR6 and CXCL12/14-CXCR4) are potential mediators of immune cell tumor infiltration[50]. Inflammatory chemokines, CCL2, CCL3, and CCL5, recruited to the tumor site monocytes exercise pro- or anti-tumoral roles after differentiating into TAMs. CCL2 also promotes tumor angiogenesis and endothelial cell survival[51]. The CC chemokine receptor type 6 (CCR6) in epithelial tissue is responsible for the recruitment of dendritic cells (DCs) to the cancer milieu[52], as observed with CCL5 as well[51]. In OC, CCR6 is associated with poor disease outcomes, as high levels of this receptor appear to contribute to tumor cell migration[53]. CC 7 chemokine ligand (CCL7) is typically associated with macrophage and eosinophil recruitment[54]. Jeong et al. observed that OC cell lines (A2780 and OVCAR-3) stimulated CCL7 production in macrophages that started to act in the TME, promoting metastasis in both cell lines[55]. CC chemokine receptor 1 (CCR1) is typically associated with monocyte recruitment; however, it is overexpressed in OC compared to normal tissue. Interestingly, increased CCR1 expression occurred in the omentum, where metastasis often occurs[56]. Table 1 shows examples of the relationship between these molecules and OC progression.
Role of interleukins and chemokines in the OC’s progression
| Interleukins | |
| IL-6 | ● OC cell growth and proliferation[37,38] ● Survival and metastasis, regulating macrophage infiltration to OC, supporting the maintenance of a favorable TME for cancer progression[41,42] |
| IL-6, IL-8 e IL-10 | ● Found in the serum of advanced OC patients at significantly higher levels than samples of early-stage OC patients, with a role in progression[45] |
| IL-8 | ● Autophagy downregulation and migration upregulation of OC cell lines[46] |
| IL-34 | ● Promotes macrophage colony formation, which directly impacts innate immunity and inflammation, causing OC progression[43] ● Related to OC’s increased growth, proliferation, and metastasis, and overexpressed after cancer cells exposition to chemotherapy[44] |
| Chemokine ligands | |
| CCL2 | ● Promotes tumor angiogenesis and endothelial cell survival[53] |
| CCL5 | ● Recruitment of DCs to the cancer medium[53] |
| CCL2, CCL3, and CCL5 | ● Recruits at the tumor site monocytes that exercise pro- or anti-tumoral roles after differentiating into TAMs[53] |
| CCL7 | ● OC cell lines (A2780 and OVCAR-3) stimulated CCL7 production in macrophages that started in the TME, promoting metastasis[57] |
| CCL20 | ● High expression in TAMs positively related to metastasis in clinical OC samples[61] |
| CCR1 | ● Normally associated with monocyte recruitment, it is overexpressed in OC compared to normal tissue[58] |
| CCR6 | ● Present in epithelial tissue and is responsible for the recruitment of DCs to the cancer milieu[54] ●In OC, it is associated with poor outcomes disease since high levels seem to contribute to tumor cell migration[55] |
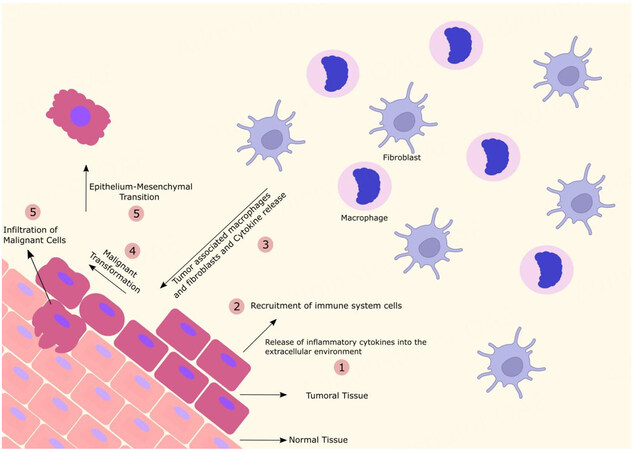
It should be noted that high expression levels of these chemokines in cancer cells help assemble immune system cells and stimulate them to cooperate with the metastasis process while modulating TME functions, thereby facilitating cancer progression[57,58]. Figure 1 represents some aspects of the ovarian TME.
Figure 1. The ovarian TME. The release of pro-inflammatory cytokines by tumor tissue makes the TME inflammatory, with the recruitment of immune cells and the differentiation of fibroblasts and macrophages into tumor-associated cells. These processes induce a malignant transformation causing epithelial-mesenchymal transition and infiltration of tumor cells favoring their progression, invasion, and metastasis.
CAFs
Conversion of normal fibroblasts in CAFs
Fibroblasts are cellular types that differ in origin and have distinct functions and anatomical locations when compared to epithelial cells (for example)[59]. They are present in the peritoneal cavity as a monolayer of mesothelial cells (MCs) lining the connective tissue with adipocytes and immune system cells[60]. In normal ovarian tissue, fibroblasts produce collagen to maintain the extracellular matrix, thereby participating in tissue homeostasis[61]. Tumor-associated fibroblasts derive from reprogrammed healthy fibroblasts or resident mesenchymal stem cells. The literature suggests that CAFs are a complex and heterogeneous population of cells[62] derived from various cell types. Definitive proof of cell origin is lacking and is based on lineage-tracing experiments[63]. Pro- and anti-tumor functions of CAFs (corroborating the information above) derive from subclassifications of CAFs, each with distinct characteristics[64,65]. In OC, subtypes CAF-S1, S2, S3, and S4 have been found in TME when the markers fibroblast activation protein (FAP), CD29 (integrin-β1), smooth muscle α-actin, fibroblast-specific protein 1, platelet-derived growth factor receptor-β, and caveolin 1 were analyzed[66].
In cancerous tissues, tumor cells influence the TME, and due to this characteristic, MCs are transformed into CAFs via mesothelial-mesenchymal transition during metastasis[67,68]. At this stage, MCs undergo major genetic reprogramming, culminating in acquiring a phenotype similar to that of CAFs. Several promoting stimuli are needed to trigger the change in MCs; however, transforming pro-fibrotic growth factor β1 (TGF-β1) is considered a critical factor in this process[69]. In response to TGF-β1, receptor-mediated signaling activates a complex network of cellular signaling pathways, including phosphatidylinositol 3-kinase/protein kinase B, nuclear factor kappa B), mitogen-activated protein kinase), c-Jun N-terminal kinase, and TGF-β- activated kinase 1[70].
During transformation, MCs lose their apical-basolateral polarity and dissociate from the peritoneal monolayer while reorganizing their actin cytoskeleton. MCs then acquire the properties of migration and invasion[71,72]. After mesothelial monolayer destruction, advancing already modified MCs to peritoneum deeper layers makes interaction with cancer cells possible[60,67,68].
CAF activation
Tumors (especially solid ones) recruit CAFs and influence them to synthesize compounds that impact various cancer aspects[73]. For CAF activation, the secretion of tumor IL-6, IL-8, and IL-1β is required[74]. The mechanism by which the transformation of normal fibroblasts in CAFs occurs is poorly understood[75,76]. However, microRNAs (miR) miR-31, miR-214, and miR-155 are related to the genesis of these cells[77]. Stanniocalcin 1 also assists in the transformation of normal fibroblasts in CAFs[78]. Overexpression of the activated signal transducer and activator of transcription 4 in OC cells induce CAF formation through wingless/integrated 7A signaling pathway activation[79]. The relationship between the overexpression of the SNAIL (Zinc Finger Protein SNAIL) family transcriptional repressor 2 (SNAI2) in fibroblasts and the transformation of fibroblasts in CAFs was observed in the 3D organotypic coculture of OC, capable of supporting cells growth[80].
CAFs’ role in metastasis
After CAF activation, increased expression of the peptide progranulin through the upregulation of α-smooth muscle actin in fibroblasts results in EMT continuation[81]. EMT initiates with cytoskeleton remodeling and loss of basolateral polarity[82]. Hence, it is speculated that the SNAIL family is related to EMT, and it was shown that SNAI2 overexpression increased cell motility in OC cell models[80]. Thus, high motility is related to EMT[82]. A series of signals can induce EMT, including the transient receptor potential of melastatin 7, by activating the PI3K/AKT signaling pathway[83]. The long non-coding RNA SRA increased migration and induced EMT through the neurogenic locus notch homolog protein 1 signaling pathway[84]. Some CAF subtypes express dikkopf-3 and activate the yes-associated protein 1/tafazzin pathway, which is associated with OC aggressive features[85]. Nevertheless, CAFs expressing stanniocalcin 1 promote cell motility and invasion in OC cell cultures (SKOV3 and HEY-T30)[78].
Xu et al. reported co-cultures of fibroblasts and OC cells, in which the tumor cells migrated more due to increased protein expression of enhancer of zeste homolog 2[86]. CAFs increased vascular endothelial growth factor (VEGF) expression, which can be activated by platelet-derived growth factor (PDGF)[87]. On the other hand, PDGF acts on its receptor to induce angiogenesis by recruiting CAFs that secrete VEGF[88]. Hence, VEGF also stimulates OC stem cells to express B cell-specific Moloney murine leukemia virus integration site 1, a molecule related to preventing cell senescence and maintaining the self-renewal capacity of cancer stem cells (CSCs)[89,90]. This finding is relevant because the population of CSCs has shown a robust ability to achieve self-renewal. These cells initiate and maintain leukemias; however, it was found that they may also participate in developing and establishing some solid tumors[91]. Furthermore, some CSCs express increased nuclear factor erythroid 2-related factor 2 activity, an essential transcription factor involved with oxidative damage protection in many cell types, including OC cells. Therefore, NRF2 may participate in the acquisition of chemoresistance by OCs, because the antioxidant role of NRF2 can potentially minimize the effects of drugs used conventionally to treat cancer[92].
CAFs are correlated with CSCs maintenance by insulin-like growth factor 1 release. IGF is related to CSCs pluripotency and OC chemoresistance[93,94]. These factors activate the IGF-1R-AKT signaling pathway, which culminates in increased expression and production of genes involved in self-renewal (OCT4/SOX2/NANOG) that promote the acquisition of resistance to chemotherapeutics and maintain CSCs[95].
CAFs participate in the expression of CXCL12/stromal cell-derived factor-1, macrophage colony-stimulating factor (CSF-1), IL-6, and CCL2/MCP-1. Thus, they stimulate TAM recruitment[61]. CAFs also induce the proliferation of cisplatin-resistant OVCAR-3 and SKOV-3 EOC cells through CXCL12/CXCR4/Wnt/β-catenin pathway activation[96]. CAFs upregulate the expression of lipoma preferred partner (LPP) in endothelial cells, which facilitates their motility. Data suggest that high LPP expression reflects poor disease outcomes for HGSOC patients[97].
CAFs as potential targets for therapeutic strategies
Given CAFs' importance for tumor progression, metastasis, and drug resistance, many therapeutic strategies target these cells and their products to fight cancer. Conventional therapy for treating OC, based on platinum derivatives, taxanes, and poly ADP-ribose polymerase inhibitors, also acts on CAFs and thus inhibits or promotes the pro-tumorigenic effects of CAFs[98]. However, it should be noted that none of these strategies is specific to CAFs, as other cell types also express or secrete similar compounds[99]. Nonetheless, targeting the depletion of the CAF population is sought by a DNA vaccine against FAP. According to Wen et al., this therapy appears effective as a tumor rejection antigen, suppressing primary colon tumor and lung metastases in treated mice, primarily through CD8(+) T cell-mediated death[100].
In this context, the studies showed that the DNA vaccine directed to murine FAP efficiently eliminated CAFs via activation of TCD8+ cells, culminating in a substantial increase in the uptake of chemotherapeutics by resistant colon and breast cancer cells[101]. Another strategy related to FAP is the construction of chimeric antigen receptor T cells used for cancer treatment. In preclinical models, chimeric antigen receptor T cells targeting CAFs FAP+ triggered a specific immune attack against CAFs, and no severe side effects were observed[102,103]. CAFs FPA+ are responsible for the production of stromal cell-derived factor 1 chemokine in the tumor stroma, which binds to CXCR4 and activates pathways that culminate in immunosuppression. CAFs FAP+ were associated with immune evasion in a human pancreatic ductal adenocarcinoma model related to CXCL12 secretion, and the depletion of these CAFs enabled the immunological control of model growth[104].
Another potential target is TGF-β because it is a primary factor in CAF activation[105]. For this reason, inhibiting the TGF-β pathway can prevent quiescent fibroblast activation. Studies demonstrated that this pathway inhibition in colorectal and urogenital cancers increased the success of therapy with anti-PD-L1 agents[106]. A phase II clinical trial investigates the combination of gemcitabine and galunisertib, a TGF-β inhibitor. To date, the trial demonstrated increased overall survival of pancreatic cancer patients treated with the drug than those that did not receive this treatment[107]. Fresolimumab, an anti-TGF-β, showed anti-tumoral activity in melanoma and renal cell carcinoma[108]. The association of this antibody with radiotherapy increased overall survival in metastatic breast cancer patients[109]. Another fundamental approach is TGF-β silencing, which reduces tumoral growth in pancreatic cancer murine models[110].
Fibroblast growth factor receptor has been used as a target against cancer[111]. Its inhibitor, erdafitinib, demonstrated potential anti-tumoral activity against metastatic urothelial carcinoma[112] and cholangiocarcinoma, as shown in a phase I trial study[113]. The same inhibitor is under study for solid tumors such as breast cancer, hepatocarcinoma, and prostate cancer[114]. AZD4547, another inhibitor, showed compelling activity in breast cancer patients in phase II trial studies[115]. However, for other malignant neoplasias, such as mesothelioma, lung, and gastric cancer, this inhibitor was not efficient[116-118]. There are no studies of CAFs in OC. In this context, one can postulate that the high heterogeneity of CAFs influences cancer therapy effectiveness. Therefore, the development of novel treatment strategies targeting these cells remains challenging. Concerning OC, it remains unknown whether OC-associated fibroblasts could be targeted for innovative therapeutic approaches.
TAMs
Macrophages are the primary effector cells in innate immunity and act in host defense, inflammation, and tissue homeostasis[119,120]. After being recruited to the TME, macrophages differentiate in TAMs, which are found in greater volume in the ovarian TME and are associated with disease progression and chemoresistance[121]. Highly plastic phenotypes and substantial diversity are characteristic of macrophages[119]. Different TAM phenotypes can be expressed according to the stimuli, among them the anti-tumorigenic M1-like and the pro-tumorigenic M2-like TAMs[121,122].
M1 macrophages act as microbicides and inhibit tumor progression through pro-inflammatory and the secretion of immunostimulant cytokines (IL-12, TNF-α, and IFN-γ). By contrast, during tumor progression, interleukins (IL-4, IL-10, and IL-13) activate macrophage differentiation in type M2, which secrete IL-4, IL-5, and IL-6, matrix metalloproteinases, pro-angiogenic factors such as VEGF, and chemokines. Thus, there is an increase in angiogenesis, matrix remodeling, and immune system suppression, which favors tumor development and metastasis[119,122-124]. However, the molecular mechanisms during the polarization of TAMs and OC metastasis are not yet fully defined[26,122].
Evidence of TAMs’ pro-tumor role
The relationship between TAMs and cancer outcomes conflict, possibly associated with macrophage heterogeneity[125]. However, in several malignancies, the elevated expression and infiltration of TAMs are involved with worse outcomes[122,126,127], as observed in OC patients[26]. It is noteworthy that the most significant infiltration of TAMs was reported for the most common OC subtype, SOC, as opposed to MOC, EC, CCC, and undifferentiated. Increased intratumoral TAMs density was directly proportional to the cancer stage[128]. High concentrations of CSF-1 in tumors are associated with poor outcomes, as it is chemotactic for macrophages and the primary lineage regulator of most macrophage populations[129]. TAMs are mostly related to SOC, and their presence can indicate tumor progression.
In the ovarian TME, the M2-like phenotype is usually expressed by TAMs and was detected using the CD163 marker in ascites samples from patients with EOC[130]. In these samples, there was a more significant number of TAMs in ascites of advanced EOC (stages III and IV) than in ascites from patients with benign or early EOC (stage I)[130]. Lan et al. showed that M2-like macrophages in the peritumoral stroma of more aggressive ovarian tumors with intense TAMs density have higher expression of CD163 than those in less aggressive tumors[125]. For SOC and MOC, the number of macrophages expressing CD68, CD163, and CD204 in borderline and malignant tumors was significantly higher than in benign tumors[123].
Evidence that macrophage recruitment from the peritoneal cavity occurs during the initial phase of metastasis supports the presence of M1 (CD68+) and M2 (CD163+) macrophages in omental samples from OC patients[131]. These macrophages promote metastasis to the omentum by secreting chemokine ligands interacting with chemokine receptors. Macrophage depletion and CCR1 inhibition have been reported to reduce colonization of the omentum by OC cells[56]. In clinical OC samples, the high expression of chemokine ligand 20 (CCL20) in TAMs and CCR6 in tumors was positively related to metastasis[59]. These findings suggest that CCL20 and CCR6 inactivation inhibit TAM-induced OC progression[59]. Curiously, it has been reported that the depletion of CD163+ Tim4+ tissue-resident macrophages in omentum prevented tumor progression and metastatic spread in a mouse model of metastatic OC[132].
Co-culture of TAMs with OC cells demonstrated pro-tumor activation interactions. These interactions include increased secretion of pro-tumor cytokines IL-6 and IL-10, lower sensitivity to carboplatin, and increased cell invasiveness[133]. STAT3 activation in macrophages was related to IL-6 and IL-10 that reactivate STAT3 in OC cells. Nevertheless, macrophage STAT3 blockage inhibited the production of these cytokines and suppressed STAT3 activation in tumor cells, suggesting that this pathway plays a central role in the interaction between tumor cells and macrophages[130].
Zeng et al. measured increased epidermal growth factor levels in M2-like TAMs and OC cell lines co-culture supernatants and enhanced cell viability, proliferation, migration, and invasion of cells associated with TAMs[134]. The expression of EMT biomarkers was upregulated, as were N-cadherin, vimentin, and epidermal growth factor receptor (EGFR)/ERK signals. However, EGFR inhibition repressed tumor growth in the co-culture of M2-like TAMs and OC cell lines system[134]. These findings illustrate how macrophages and their secreted growth factors can promote tumor progression.
Therapeutic strategies involving TAMs
TAMs’ pro-tumor role occurs through the secretion of cytokines, chemokines, and enzymes that contribute to the progression, chemoresistance, and metastasis of OC. TAMs also inhibit the responsiveness of immune cells (lymphocytes, natural killer cells, and DCs), which leads to immunosuppression[121,129]. In this context, therapeutic alternatives to combat TAMs represent novel and effective treatment possibilities. Examples of these new strategies include the ablation of macrophage formation from the TME, tested for pro-tumorigenic M2-like phenotype, inhibition of pathways involving chemokine receptors and chemokine ligands that have been linked to TAM-associated OC progression, and inhibition of factors capable of stimulating the overactivation of TAMs, such as CFS-1 receptors blockade[53,56,129,130,134]. Detailed studies in this field should be performed to support therapy against OC.
CONCLUSIONS
The TME has received substantial attention regarding its role in the development, immune evasion, and metastasis of different malignancies, including OC. The observation relies, at least in part, on the presence of epithelial or immune system cells and substances that trigger the body's immune response and support the mechanisms of OC cells' invasiveness, proliferation, migration, and chemoresistance. As highlighted in the present review, factors that participate in inflammation and immune response, such as the high presence of interleukins (IL-6, IL-8, IL-10, IL-34), chemokine ligands (CCL7), and chemokine receptors (CCR1 and CCR6) are OC progression-associated components. Fibroblast differentiation in CAFs relies on the participation of interleukins (IL-6, IL-8, and IL-1β) secreted by the tumor. CAFs potentialize ovarian tumor progression through the secretion of VEGF and other factors (i.e., CSF-1 and IL-6), which stimulate TAM recruitment. Macrophages associated with the ovarian tumor may present the pro-tumorigenic phenotype. In this case, tumor metastasis is enabled by interleukin secretion, the expression of chemokine ligands and their receptors, and EMT factors. Conventional therapies against OC are based on surgery, radiation, and cytotoxic chemotherapy and often do not show suitable long-term results. The inhibition and redefinition of these TME components might improve OC outcomes, contributing to more prolonged survival.
DECLARATIONS
AcknowledgmentsThe authors thank the fellowships provided by CNPq and FAPES.
Authors’ contributionsConception and design of the study, information analysis, and interpretation: Souza JC, Junior RSR, Pimenta TM, Martins BS, Rangel LBA
Chapter review: Rangel LBA
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023
REFERENCES
1. American Cancer Society (ACS). Key statistics for ovarian cancer. Available from: https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html [Last accessed on 16 Feb 2022].
3. Stewart C, Ralyea C, Lockwood S. Ovarian cancer: an integrated review. Semin Oncol Nurs 2019;35:151-6.
4. Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol 2017;41:3-14.
6. Paes MF, Daltoé RD, Madeira KP, et al. A retrospective analysis of clinicopathological and prognostic characteristics of ovarian tumors in the State of Espírito Santo, Brazil. J Ovarian Res 2011;4:14.
7. Torres MP, Ponnusamy MP, Lakshmanan I, Batra SK. Immunopathogenesis of ovarian cancer. Minerva Med 2009;100:385-400.
8. World Health Organization. Female genital tumours: WHO classification of tumours 5th ed. volume 4 IARC. Lyon, France: WHO; 2020.
10. Kossaï M, Leary A, Scoazec JY, Genestie C. Ovarian cancer: a heterogeneous disease. Pathobiology 2018;85:41-9.
11. Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev Cancer 2010;10:803-8.
12. Kroeger PT Jr, Drapkin R. Pathogenesis and heterogeneity of ovarian cancer. Curr Opin Obstet Gynecol 2017;29:26-34.
13. Kujawa KA, Lisowska KM. Rak jajnika-od biologii do kliniki [ovarian cancer-from biology to clinic]. Postepy Hig Med Dosw 2015;69:1275-90.
14. Kurman RJ, Shih IeM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 2010;34:433-43.
15. Cheasley D, Wakefield MJ, Ryland GL, et al. The molecular origin and taxonomy of mucinous ovarian carcinoma. Nat Commun 2019;10:3935.
17. Orr B, Edwards RP. Diagnosis and treatment of ovarian cancer. Hematol Oncol Clin N Am 2018;32:943-64.
18. Jia D, Nagaoka Y, Katsumata M, Orsulic S. Inflammation is a key contributor to ovarian cancer cell seeding. Sci Rep 2018;8:12394.
19. Kisielewski R, Tołwińska A, Mazurek A, Laudański P. Inflammation and ovarian cancer-current views. Ginekol Pol 2013;84:293-7.
21. Savant SS, Sriramkumar S, O’Hagan HM. The role of inflammation and inflammatory mediators in the development, progression, metastasis, and chemoresistance of epithelial ovarian cancer. Cancers 2018;10:251.
22. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012;21:309-22.
23. Wang X, Yung MMH, Sharma R, et al. Epigenetic silencing of miR-33b promotes peritoneal metastases of ovarian cancer by modulating the TAK1/FASN/CPT1A/NF-κB axis. Cancers 2021;13:4795.
24. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013;19:1423-37.
26. Jiang Y, Wang C, Zhou S. Targeting tumor microenvironment in ovarian cancer: premise and promise. Biochim Biophys Acta Rev Cancer 2020;1873:188361.
27. Qian J, LeSavage BL, Hubka KM, et al. Cancer-associated mesothelial cells promote ovarian cancer chemoresistance through paracrine osteopontin signaling. J Clin Invest 2021;131:e146186.
28. Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature 2004;432:332-7.
29. Zhang AW, McPherson A, Milne K, et al. Interfaces of malignant and immunologic clonal dynamics in ovarian cancer. Cell 2018;173:1755-69.e22.
30. Lee W, Ko SY, Mohamed MS, Kenny HA, Lengyel E, Naora H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med 2019;216:176-94.
32. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017;14:399-416.
33. Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm 2016;2016:6058147.
34. Shan W, Liu J. Inflammation: a hidden path to breaking the spell of ovarian cancer. Cell Cycle 2009;8:3107-11.
35. Amer H, Kartikasari AER, Plebanski M. Elevated interleukin-6 levels in the circulation and peritoneal fluid of patients with ovarian cancer as a potential diagnostic biomarker: a systematic review and meta-analysis. J Pers Med 2021;11:1335.
36. Zhang H, Wang Z, Wang F, Wang C, Zhang H. IL-6 and IL-8 are involved in JMJD2A-regulated malignancy of ovarian cancer cells. Arch Biochem Biophys 2020;684:108334.
37. Szulc-Kielbik I, Kielbik M, Nowak M, Klink M. The implication of IL-6 in the invasiveness and chemoresistance of ovarian cancer cells. Systematic review of its potential role as a biomarker in ovarian cancer patients. Biochim Biophys Acta Rev Cancer 2021;1876:188639.
38. Jordan KR, Sikora MJ, Slansky JE, et al. The capacity of the ovarian cancer tumor microenvironment to integrate inflammation signaling conveys a shorter disease-free interval. Clin Cancer Res 2020;26:6362-73.
39. Browning L, Patel MR, Horvath EB, Tawara K, Jorcyk CL. IL-6 and ovarian cancer: inflammatory cytokines in promotion of metastasis. Cancer Manag Res 2018;10:6685-93.
40. Jiang B, Zhu SJ, Xiao SS, Xue M. MiR-217 inhibits M2-Like macrophage polarization by suppressing secretion of interleukin-6 in ovarian cancer. Inflammation 2019;42:1517-29.
41. Wang B, Xu W, Tan M, Xiao Y, Yang H, Xia TS. Integrative genomic analyses of a novel cytokine, interleukin-34 and its potential role in cancer prediction. Int J Mol Med 2015;35:92-102.
42. Endo H, Hama N, Baghdadi M, et al. Interleukin-34 expression in ovarian cancer: a possible correlation with disease progression. Int Immunol 2020;32:175-86.
43. Nowak M, Glowacka E, Szpakowski M, et al. Proinflammatory and immunosuppressive serum, ascites and cyst fluid cytokines in patients with early and advanced ovarian cancer and benign ovarian tumors. Neuro Endocrinol Lett 2010;31:375-83.
44. Thongchot S, Jamjuntra P, Therasakvichya S, et al. Interleukin-8 released by cancer-associated fibroblasts attenuates the autophagy and promotes the migration of ovarian cancer cells. Int J Oncol 2021;58:14.
45. Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 2006;354:610-21.
47. Sokol CL, Luster AD. The chemokine system in innate immunity. Cold Spring Harb Perspect Biol 2015;7:a016303.
48. Letourneur D, Danlos FX, Marabelle A. Chemokine biology on immune checkpoint-targeted therapies. Eur J Cancer 2020;137:260-71.
49. Singha B, Gatla HR, Vancurova I. Transcriptional regulation of chemokine expression in ovarian cancer. Biomolecules 2015;5:223-43.
50. Hornburg M, Desbois M, Lu S, et al. Single-cell dissection of cellular components and interactions shaping the tumor immune phenotypes in ovarian cancer. Cancer Cell 2021;39:928-44.e6.
51. Poeta V, Massara M, Capucetti A, Bonecchi R. Chemokines and chemokine receptors: new targets for cancer immunotherapy. Front Immunol 2019;10:379.
52. Ito T, Carson WF 4th, Cavassani KA, Connett JM, Kunkel SL. CCR6 as a mediator of immunity in the lung and gut. Exp Cell Res 2011;317:613-9.
53. Liu W, Wang W, Wang X, Xu C, Zhang N, Di W. Cisplatin-stimulated macrophages promote ovarian cancer migration via the CCL20-CCR6 axis. Cancer Lett 2020;472:59-69.
54. Cheng JW, Sadeghi Z, Levine AD, et al. The role of CXCL12 and CCL7 chemokines in immune regulation, embryonic development, and tissue regeneration. Cytokine 2014;69:277-83.
55. Jeong M, Wang YY, Choi JY, Lim MC, Choi JH. CC chemokine ligand 7 derived from cancer-stimulated macrophages promotes ovarian cancer cell invasion. Cancers 2021;13:2745.
56. Krishnan V, Tallapragada S, Schaar B, et al. Omental macrophages secrete chemokine ligands that promote ovarian cancer colonization of the omentum via CCR1. Commun Biol 2020;3:524.
57. Mao X, Xu J, Wang W, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer 2021;20:131.
58. Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol 2019;12:76.
59. Plikus MV, Wang X, Sinha S, et al. Fibroblasts: origins, definitions, and functions in health and disease. Cell 2021;184:3852-72.
61. Sun W, Fu S. Role of cancer-associated fibroblasts in tumor structure, composition and the microenvironment in ovarian cancer. Oncol Lett 2019;18:2173-8.
62. Öhlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017;214:579-96.
63. Sahai E, Astsaturov I, Cukierman E, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer 2020;20:174-86.
64. Zhang M, Chen Z, Wang Y, Zhao H, Du Y. The role of cancer-associated fibroblasts in ovarian cancer. Cancers 2022;14:2637.
65. Xu J, Fang Y, Chen K, et al. Single-cell RNA sequencing reveals the tissue architecture in human high-grade serous ovarian cancer. Clin Cancer Res 2022;28:3590-602.
66. Givel AM, Kieffer Y, Scholer-Dahirel A, et al. miR200-regulated CXCL12β promotes fibroblast heterogeneity and immunosuppression in ovarian cancers. Nat Commun 2018;9:1056.
67. Sandoval P, Jiménez-Heffernan JA, Rynne-Vidal Á, et al. Carcinoma-associated fibroblasts derive from mesothelial cells via mesothelial-to-mesenchymal transition in peritoneal metastasis. J Pathol 2013;231:517-31.
68. Rynne-Vidal A, Jiménez-Heffernan JA, Fernández-Chacón C, López-Cabrera M, Sandoval P. The mesothelial origin of carcinoma associated-fibroblasts in peritoneal metastasis. Cancers 2015;7:1994-2011.
69. Loureiro J, Aguilera A, Selgas R, et al. Blocking TGF-β1 protects the peritoneal membrane from dialysate-induced damage. J Am Soc Nephrol 2011;22:1682-95.
70. López-Cabrera M. Mesenchymal conversion of mesothelial cells is a key event in the pathophysiology of the peritoneum during peritoneal dialysis. Adv Med 2014;2014:473134.
71. Yáñez-Mó M, Lara-Pezzi E, Selgas R, et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med 2003;348:403-13.
72. Ruiz-Carpio V, Sandoval P, Aguilera A, et al. Genomic reprograming analysis of the mesothelial to mesenchymal transition identifies biomarkers in peritoneal dialysis patients. Sci Rep 2017;7:44941.
74. Schauer IG, Sood AK, Mok S, Liu J. Cancer-associated fibroblasts and their putative role in potentiating the initiation and development of epithelial ovarian cancer. Neoplasia 2011;13:393-405.
75. Calvo F, Ege N, Grande-Garcia A, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol 2013;15:637-46.
76. Zhang Y, Tang H, Cai J, et al. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett 2011;303:47-55.
77. Mitra AK, Zillhardt M, Hua Y, et al. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov 2012;2:1100-8.
78. Yang Y, Yin S, Li S, Chen Y, Yang L. Stanniocalcin 1 in tumor microenvironment promotes metastasis of ovarian cancer. Onco Targets Ther 2019;12:2789-98.
79. Zhao L, Ji G, Le X, et al. An integrated analysis identifies STAT4 as a key regulator of ovarian cancer metastasis. Oncogene 2017;36:3384-96.
80. Yang Z, Yang X, Xu S, et al. Reprogramming of stromal fibroblasts by SNAI2 contributes to tumor desmoplasia and ovarian cancer progression. Mol Cancer 2017;16:163.
81. Yeung TL, Leung CS, Wong KK, et al. TGF-β modulates ovarian cancer invasion by upregulating CAF-derived versican in the tumor microenvironment. Cancer Res 2013;73:5016-28.
82. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014;15:178-96.
83. Liu L, Wu N, Wang Y, et al. TRPM7 promotes the epithelial-mesenchymal transition in ovarian cancer through the calcium-related PI3K/AKT oncogenic signaling. J Exp Clin Cancer Res 2019;38:106.
84. Kim LK, Park SA, Yang Y, Kim YT, Heo TH, Kim HJ. LncRNA SRA mediates cell migration, invasion, and progression of ovarian cancer via NOTCH signaling and epithelial-mesenchymal transition. Biosci Rep 2021;41:BSR20210565.
85. Ferrari N, Ranftl R, Chicherova I, et al. Dickkopf-3 links HSF1 and YAP/TAZ signalling to control aggressive behaviours in cancer-associated fibroblasts. Nat Commun 2019;10:130.
86. Xu L, Deng Q, Pan Y, et al. Cancer-associated fibroblasts enhance the migration ability of ovarian cancer cells by increasing EZH2 expression. Int J Mol Med 2014;33:91-6.
87. Gomes FG, Nedel F, Alves AM, Nör JE, Tarquinio SB. Tumor angiogenesis and lymphangiogenesis: tumor/endothelial crosstalk and cellular/microenvironmental signaling mechanisms. Life Sci 2013;92:101-7.
88. Ferrara N. Pathways mediating VEGF-independent tumor angiogenesis. Cytokine Growth Factor Rev 2010;21:21-6.
89. Zhao Q, Gui T, Qian Q, Li L, Shen K. B-cell-specific Moloney murine leukemia virus integration site 1: potential stratification factor and therapeutic target for epithelial ovarian cancer. Onco Targets Ther 2016;9:5203-8.
90. Huang X, Cho S, Spangrude GJ. Hematopoietic stem cells: generation and self-renewal. Cell Death Differ 2007;14:1851-9.
91. Trumpp A, Wiestler OD. Mechanisms of disease: cancer stem cells-targeting the evil twin. Nat Clin Pract Oncol 2008;5:337-47.
92. Tossetta G, Marzioni D. Natural and synthetic compounds in ovarian cancer: a focus on NRF2/KEAP1 pathway. Pharmacol Res 2022;183:106365.
93. Jeter CR, Yang T, Wang J, Chao HP, Tang DG. Concise review: NANOG in cancer stem cells and tumor development: an update and outstanding questions. Stem Cells 2015;33:2381-90.
94. Denduluri SK, Idowu O, Wang Z, et al. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis 2015;2:13-25.
95. Singh RK, Dhadve A, Sakpal A, De A, Ray P. An active IGF-1R-AKT signaling imparts functional heterogeneity in ovarian CSC population. Sci Rep 2016;6:36612.
96. Zhang F, Cui JY, Gao HF, et al. Cancer-associated fibroblasts induce epithelial-mesenchymal transition and cisplatin resistance in ovarian cancer via CXCL12/CXCR4 axis. Future Oncol 2020;16:2619-33.
97. Leung CS, Yeung TL, Yip KP, et al. Cancer-associated fibroblasts regulate endothelial adhesion protein LPP to promote ovarian cancer chemoresistance. J Clin Invest 2018;128:589-606.
98. Eckert MA, Orozco C, Xiao J, Javellana M, Lengyel E. The effects of chemotherapeutics on the ovarian cancer microenvironment. Cancers 2021;13:3136.
99. Belhabib I, Zaghdoudi S, Lac C, Bousquet C, Jean C. Extracellular matrices and cancer-associated fibroblasts: targets for cancer diagnosis and therapy? Cancers 2021;13:3466.
100. Wen Y, Wang CT, Ma TT, et al. Immunotherapy targeting fibroblast activation protein inhibits tumor growth and increases survival in a murine colon cancer model. Cancer Sci 2010;101:2325-32.
101. Loeffler M, Krüger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest 2006;116:1955-62.
102. Kakarla S, Chow KK, Mata M, et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol Ther 2013;21:1611-20.
103. Wang LC, Lo A, Scholler J, et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res 2014;2:154-66.
104. Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA 2013;110:20212-17.
105. Neuzillet C, de Gramont A, Tijeras-Raballand A, et al. Perspectives of TGF-β inhibition in pancreatic and hepatocellular carcinomas. Oncotarget 2014;5:78-94.
106. Mariathasan S, Turley SJ, Nickles D, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554:544-8.
107. Melisi D, Garcia-Carbonero R, Macarulla T, et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br J Cancer 2018;119:1208-14.
108. Morris JC, Tan AR, Olencki TE, et al. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFβ) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS One 2014;9:e90353.
109. Formenti SC, Lee P, Adams S, et al. Focal irradiation and systemic TGFβ blockade in metastatic breast cancer. Clin Cancer Res 2018;24:2493-504.
110. Schlingensiepen KH, Jaschinski F, Lang SA, et al. Transforming growth factor-beta 2 gene silencing with trabedersen (AP 12009) in pancreatic cancer. Cancer Sci 2011;102:1193-200.
111. Haugsten EM, Wiedlocha A, Olsnes S, Wesche J. Roles of fibroblast growth factor receptors in carcinogenesis. Mol Cancer Res 2010;8:1439-52.
112. Loriot Y, Necchi A, Park SH, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med 2019;381:338-48.
113. Bahleda R, Italiano A, Hierro C, et al. Multicenter phase I study of erdafitinib (JNJ-42756493), oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced or refractory solid tumors. Clin Cancer Res 2019;25:4888-97.
114. Ganguly D, Chandra R, Karalis J, et al. Cancer-associated fibroblasts: versatile players in the tumor microenvironment. Cancers 2020;12:2652.
115. Seckl M, Badman PD, Liu X, et al. RADICAL trial: A phase Ib/IIa study to assess the safety and efficacy of AZD4547 in combination with either anastrozole or letrozole in ER positive breast cancer patients progressing on these aromatase inhibitors (AIs). JCO 2017;35:1059.
116. Lam WS, Creaney J, Chen FK, et al. A phase II trial of single oral FGF inhibitor, AZD4547, as second or third line therapy in malignant pleural mesothelioma. Lung Cancer 2020;140:87-92.
117. Aggarwal C, Redman MW, Lara PN Jr, et al. SWOG S1400D (NCT02965378), a phase ii study of the fibroblast growth factor receptor inhibitor AZD4547 in previously treated patients with fibroblast growth factor pathway-activated stage IV squamous cell lung cancer (lung-MAP substudy). J Thorac Oncol 2019;14:1847-52.
118. Van Cutsem E, Bang YJ, Mansoor W, et al. A randomized, open-label study of the efficacy and safety of AZD4547 monotherapy versus paclitaxel for the treatment of advanced gastric adenocarcinoma with FGFR2 polysomy or gene amplification. Ann Oncol 2017;28:1316-24.
119. Oishi Y, Manabe I. Macrophages in inflammation, repair and regeneration. Int Immunol 2018;30:511-28.
120. Petty AJ, Yang Y. Tumor-associated macrophages: implications in cancer immunotherapy. Immunotherapy 2017;9:289-302.
121. Nowak M, Klink M. The role of tumor-associated macrophages in the progression and chemoresistance of ovarian cancer. Cells 2020;9:1299.
122. Luo Z, Wang Q, Lau WB, et al. Tumor microenvironment: the culprit for ovarian cancer metastasis? Cancer Lett 2016;377:174-82.
123. Kawamura K, Komohara Y, Takaishi K, Katabuchi H, Takeya M. Detection of M2 macrophages and colony-stimulating factor 1 expression in serous and mucinous ovarian epithelial tumors. Pathol Int 2009;59:300-5.
124. Bellora F, Castriconi R, Dondero A, et al. TLR activation of tumor-associated macrophages from ovarian cancer patients triggers cytolytic activity of NK cells. Eur J Immunol 2014;44:1814-22.
125. Lan C, Huang X, Lin S, et al. Expression of M2-polarized macrophages is associated with poor outcomes for advanced epithelial ovarian cancer. Technol Cancer Res Treat 2013;12:259-67.
126. Hashimoto A, Sarker D, Reebye V, et al. Upregulation of C/EBPα inhibits suppressive activity of myeloid cells and potentiates antitumor response in mice and patients with cancer. Clin Cancer Res 2021;27:5961-78.
127. Wagner J, Rapsomaniki MA, Chevrier S, et al. A Single-cell atlas of the tumor and immune ecosystem of human breast cancer. Cell 2019;177:1330-45.e18.
128. Zhang M, He Y, Sun X, et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res 2014;7:19.
129. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014;41:49-61.
130. Takaishi K, Komohara Y, Tashiro H, et al. Involvement of M2-polarized macrophages in the ascites from advanced epithelial ovarian carcinoma in tumor progression via Stat3 activation. Cancer Sci 2010;101:2128-36.
131. Olalekan S, Xie B, Back R, Eckart H, Basu A. Characterizing the tumor microenvironment of metastatic ovarian cancer by single-cell transcriptomics. Cell Rep 2021;35:109165.
132. Etzerodt A, Moulin M, Doktor TK, et al. Tissue-resident macrophages in omentum promote metastatic spread of ovarian cancer. J Exp Med 2020;217:e20191869.
133. Raghavan S, Mehta P, Xie Y, Lei YL, Mehta G. Ovarian cancer stem cells and macrophages reciprocally interact through the WNT pathway to promote pro-tumoral and malignant phenotypes in 3D engineered microenvironments. J Immunother Cancer 2019;7:190.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Souza JC, Junior RSR, Pimenta TM, Martins BS, Rangel LBA. The role of pro-inflammatory components, carcinoma-associated fibroblasts, and tumor-associated macrophages in ovarian cancer progression and metastasis. J Cancer Metastasis Treat 2023;9:3. http://dx.doi.org/10.20517/2394-4722.2022.91
AMA Style
Souza JC, Junior RSR, Pimenta TM, Martins BS, Rangel LBA. The role of pro-inflammatory components, carcinoma-associated fibroblasts, and tumor-associated macrophages in ovarian cancer progression and metastasis. Journal of Cancer Metastasis and Treatment. 2023; 9(1): 3. http://dx.doi.org/10.20517/2394-4722.2022.91
Chicago/Turabian Style
Souza, Josiany Carlos de, Roberto Silva Ribeiro Junior, Tatiana Massariol Pimenta, Bárbara da Silva Martins, Leticia Batista Azevedo Rangel. 2023. "The role of pro-inflammatory components, carcinoma-associated fibroblasts, and tumor-associated macrophages in ovarian cancer progression and metastasis" Journal of Cancer Metastasis and Treatment. 9, no.1: 3. http://dx.doi.org/10.20517/2394-4722.2022.91
ACS Style
Souza, JC.; Junior RSR.; Pimenta TM.; Martins BS.; Rangel LBA. The role of pro-inflammatory components, carcinoma-associated fibroblasts, and tumor-associated macrophages in ovarian cancer progression and metastasis. J. Cancer. Metastasis. Treat. 2023, 9, 3. http://dx.doi.org/10.20517/2394-4722.2022.91
About This Article
Copyright
Data & Comments
Data

 Cite This Article 9 clicks
Cite This Article 9 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.