Recent advances in detecting premalignant pancreatic cysts
Abstract
The incidental discovery of pancreatic cysts in asymptomatic patients is on the rise due to the widespread use of cross-sectional imaging. The challenge in the management of pancreatic cysts is in distinguishing those with malignant potentials, like mucinous pancreatic cysts, from non-mucinous cysts that have negligible malignant potentials. Similarly, it can be difficult to identify mucinous cysts that harbour high-grade dysplasia or early cancer. This review focuses on the recent advances in detecting pancreatic cancer and cysts with premalignant potential.
Keywords
INTRODUCTION
Pancreatic cysts are a common discovery in clinical practice. The incidence of asymptomatic pancreatic cysts is expected to increase due to the widespread use of cross-sectional imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) scans[1]. Pancreatic cancer is the seventh leading cause of cancer-related death worldwide; the survival rate after five years is only 7%[2]. Some pancreatic cysts can be cancerous[3]. The estimated risk of developing pancreatic cancer after seven years is 3%[4]. The estimated prevalence of asymptomatic pancreatic cysts is 1.8% for patients older than 45 years[4].
The management of pancreatic cystic lesions (PCLs) often poses a dilemma. Some PCLs carry a high risk of malignant transformation, whereas other cysts are benign with a negligible risk of malignant transformation[5]. Differentiating between malignant and non-malignant PCLs is crucial for future management[6]. Another challenge in the management of PCLs is distinguishing cysts that need close monitoring from others for which the patient could be safely discharged.
Pathologically, PCLs include a heterogenous group of cysts, all of which have diverse clinical, radiological, and pathological features, Table 1 and Figure 1. Cysts like lymphoepithelial cysts, pseudocysts, and serous cysts are considered benign PCLs. Mucinous pancreatic cysts refer to intraductal papillary mucinous neoplasms (IPMNs) and mucinous cyst neoplasms (MCNs) which are regarded as premalignant[10]. IPMNs are characterised by the intraductal papillary proliferation of mucin-producing cells, which results in the cystic dilatation of pancreatic ducts[11]. They are further divided into main-duct IPMNs (MD-IPMNs), branch-duct IPMNs (BD-IPMNs), and mixed-type IPMNs.
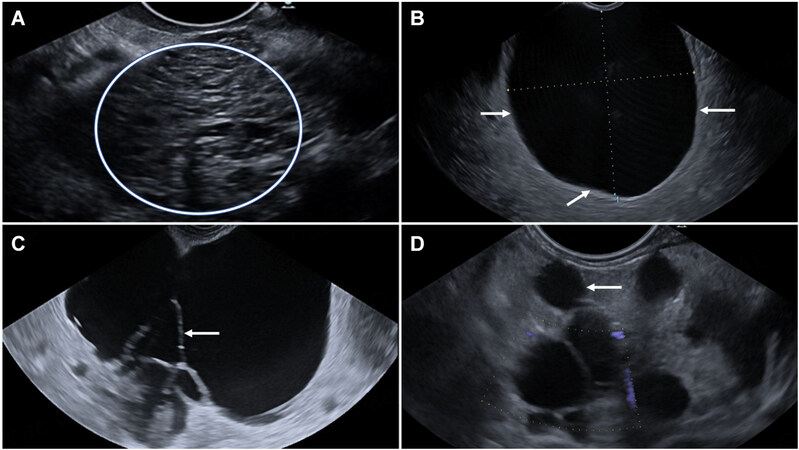
Figure 1. Endoscopic ultrasound imaging of (A) A microcystic lesion (circle) in the tail of the pancreas consistent with serous cystadenoma (SCA). (B) A large uniocular thin-walled cyst (arrows) measuring 33 mm × 27 mm in the body of pancreas. The cyst is uniformly anechoic with no solid component. The cyst has no evident communication with the pancreatic duct on the static image and therefore is consistent with the definition of a mucinous cystic neoplasm (MCN). (C) Multiseptated (arrow) thin-walled cyst in the head and neck of the pancreas. The cyst communicates with the non-dilated pancreatic duct, but this is not evident on the static image, consistent with the definition of a branch-duct IPMN. (D) Another cluster of grape-like cysts (small multiseptated cysts) communicating with a dilated PD (arrow), consistent with mixed main-duct and branch-duct IPMNs. Patient is on images surveillance.
| Pseudocyst | SCN | MCN | IPMN | |
| Age, years | Any age with a history of pancreatitis | 40-60 | 40-60 | 50-60 |
| Gender | M > F | F > M | Exclusively in women | M > F |
| Location | Pancreatic tail | More common in head | Body and tail | MD-IPMN: Head BD-IPMN: Mainly uncinate, sometimes multifocal |
| Cyst size | variable | < 2 cm | < 2 cm | Variable |
| Radiological | Thickened wall, non-septate, unilocular, anechoic lesion with debris | Multilocular, lobulated with enhanced septa | Mostly unilocular with smooth wall | MD-IPMN: cystic formation with nodules and segmental or diffuse dilatation of MPD BD: Lateral branches of the pancreas with focal multicystic lesions separated by internal septa and connected to MPD |
| Malignant potentials | Negligible | Negligible | 10%-15% | 26%-60% |
| Biochemistry | Amylase > 250 U/L Low CEA High glucose | Low amylase CEA < 5 ng/mL Elevated glucose | Low amylase CEA > 192 ng/mL Low glucose | High amylase Glucose < 50 mg/dL CEA > 192 ng/mL |
Some pancreatic tumours exhibit cystic degeneration. These include solid pseudopapillary neoplasms, cystic neuroendocrine tumours, and ductal adenocarcinomas[12]. Individuals who have an IPMN are at an increased risk of developing cancer, with 57%-92% of malignant transformations seen in MD-IPMNs and 6%-46% in BD-IPMNs. Certain clinical features, such as jaundice, weight loss, and obstructive liver blood tests, and imaging features such as a dilated main pancreatic duct (MPD) diameter > 10 mm and/or mural nodules indicate a high risk of progression to malignancy[13].
There are certain well-recognised worrisome features that raise the suspicion of an underlying malignant potential. These include a cyst size ≥ 3 cm, enhanced mural nodules > 5 mm, thickened (enhancing) cyst walls, a main pancreatic duct (MPD) calibre > 5 mm, abrupt changes in the MPD calibre with distal pancreatic atrophy, associated lymphadenopathy, elevated serum CA19-9, and a rapid rate of cyst growth
Several studies have followed up on patients with pancreatic cysts to assess malignant transformation
Summary of follow-up studies of patients with pancreatic cysts
| Author and year | Number of patients | Average age of patients | Average cyst size, mm | Cyst types | Follow-up, months | Malignant neoplastic changes or referred to surgery |
| Lee et al., 2007[17] | 45 | 63 | 13-22 | BD-IPMN | 27 | 4.4% |
| Gonzalez et al., 2012[18] | 145 | 68 | 22 | NA | 72 | 5% |
| Lawson et al., 2013[16] | 767 | 67 | NA | 78% BD-IPMN, 9% Mixed-type IPMN, 7% Serous cystadenoma, 4% MCN, 2% MD-IPMN | 48 | 9.65% |
| Nougaret et al., 2014[19] | 301 | 64 | 155 | Mainly BD-IPMN | 45 | 12% |
| Lekkerkerker et al., 2015[20] | 132 | NA | NA | Mixed-type IPMN, BD-IPMN, MCN, NET, SCA, Inflammatory cyst, Lymphangioma | 31 | 8% (referred for surgery), 0% malignancy or high-grade dysplasia |
| Broughton et al., 2016[21] | 450 | 66 | 18 | MCN (most common), IPMNs, SCA, NET and Others | 17 | 1.1% |
| Hisada et al., 2017[15] | 526 | 70 months | IPMN-16.6 Non-neoplastic cyst-12.3 | IPMN (263) and Non-neoplastic cysts (263) | 58 | Pancreatic cancer in 4% for IPMN and 0% for non-neoplastic cysts |
| Pak et al., 2017[22] | 227 | 65 | 20 for BD-IPMN, 20 for MD-IPMN, 25 for mixed-type IPMN | 84% BD-IPMN, 11% MD-IPMN, and 5% mixed-type IPMN | 56 | 3% neoplastic changes (1.8% high-grade dysplasia and 1.2% Invasive carcinoma) |
| Ohno et al., 2018[23] | 664 | 66 | 16.6 | BD-IPMN | 34 | 14.5% |
| Lee et al., 2021[24] | 982 | 68 | 12 | BD-IPMN (with no significant changes in the first 5 years) | 96 | 1% 29% high stigmata and worrisome features |
METHODS OF DIAGNOSING PANCREATIC CYSTS
CT and MRI scans
CT and MRI are the most used techniques in diagnosing and assessing pancreatic cysts. Although CT and MRI scans can be useful in the initial diagnosis and management of pancreatic cysts[25], alone, neither is adequate for fully characterising PCLs or for differentiating mucinous cyst neoplasms from macrocystic serous cyst neoplasms[26].
The accuracy of CT scans in diagnosing PCLs has been reported as 39%-61.4%, while its accuracy in differentiating benign from malignant lesions is 61.9%-80%[27]. One of the drawbacks of CT scans, particularly for patients requiring surveillance, is the repeated exposure to ionizing radiation, which increases cancer risk[28].
MRI is the technique of choice for investigating PCLs because it is less invasive and more sensitive than CT. It has better accuracy in diagnosing PCLs (50%-80%) and in differentiating benign from malignant PCLs (55.6%-87%)[27]. MRI is better at detecting communication between the pancreatic duct and a PCL as well as identifying mural nodules and septations[14]. It is also useful for identifying single and multiple PCLs, including multifocal BD-IPMNs[28].
Endoscopic ultrasound
Endoscopic ultrasound (EUS) has evolved during the last thirty years from a diagnostic procedure only to both a diagnostic and a therapeutic procedure[29]. EUS is performed to further stratify PCLs if any worrisome features (as outlined above) are reported in the cross-sectional imaging. An EUS assessment with or without sampling adds more diagnostic value than CT or MRI[26]. Pancreatic cysts of 20 mm in size or less are detected better by EUS[30]. In contrast to three-dimensional techniques such as CT/MRI, EUS is a two-dimensional technique that helps to identify adverse features those other techniques may fail to detect. Additionally, Doppler EUS or contrast-enhanced harmonic EUS reveals increases in internal vascularity, which may help to differentiate cystic NET lesions from other PCLs[31].
Although it provides better imaging capability than CT and MRI scans, EUS alone is insufficient for differentiating between benign and malignant IPMNs[32]. The advantage of EUS is that it can be combined with other techniques to improve diagnostic accuracy, for example, EUS fine-needle aspiration (EUS-FNA) or other newly developed tools like through-the-needle micro-forceps biopsy (TTNB), or confocal laser endomicroscopy (CLE).
EUS-FNA and cystic fluid analysis
EUS-FNA improves diagnostic accuracy by obtaining fluid samples for cytological, chemical, and molecular assessments[33]. EUS-FNA can differentiate mucinous from non-mucinous lesions and help to identify high-risk features[34].
Cytology
The identification of malignancy during cytological assessment using EUS-FNA was reported to have a sensitivity of 25%-88% and a specificity of 83%-99%[32]. The occurrence of macrophages, histiocytes, and neutrophils in a cytological assessment suggests a pseudocyst, while the presence of mucin indicates an MCN. Although not commonly seen, glycogen-rich cuboidal cells are diagnostic of SCA[32]. The reported sensitivity in the literature in identifying MCNs was 54%-63% and the specificity was 88%-93%[35,36].
Tumour markers
Various tumour markers have been used to differentiate between mucinous and non-mucinous PCLs and between malignant and benign PCLs. Carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 19-9, CA 72-4, and CA72-4 have been reported to present in higher levels in mucinous cysts and can aid in discriminating between mucinous and non-mucinous PCLs. With a CA 72-4 cut-off level of 3.32 ng/mL, the sensitivity, specificity, and accuracy in differentiating mucinous cysts were 80%, 69.5%, and 73.6%, respectively[37]. Another study used a cut-off of 7 U/mL with a sensitivity of 80%, a specificity of 61%, and an accuracy of 72%[38]. A meta-analysis using a CA 19-9 cut-off of 35-45 U/mL in detecting mucinous PCLs produced a sensitivity of 47% and specificity of 88%[39]. Due to CA 19-9 being a constituent of normal pancreatic digestive juices, its concentration might be similar in pseudocysts and mucinous lesions. This limits the diagnostic potential of CA 19-9, particularly when the study population includes patients with pseudocysts. However, a high level of CA 19-9 should always warrant the suspicion of malignant or potentially malignant pancreatic cystic lesions.
A CEA cut-off of 192 ng/mL is used in clinical practice to diagnose mucinous pancreatic cysts[40]. A recent meta-analysis found that a cut-off of 192 ng/mL was highly specific (88.6%) for differentiating mucinous from non-mucinous cysts and had a sensitivity of 60.4%[41].
Biochemical markers
Intra-cystic glucose measurement has yielded promising results in differentiating mucinous from non-mucinous cysts. In a multicentre study, a glucose concentration of ≤ 25 mg/dL had a sensitivity of 88.1% and a specificity of 91.2% in detecting mucinous pancreatic cysts[42].
Although amylase has been studied more widely than lipase, it has been noted that both have no useful diagnostic potential for identifying cysts with a malignant tendency[33]. If a pseudocyst has been excluded, a high level of amylase may indicate an IPMN because it suggests a communication between the cyst and the pancreatic duct.
Furthermore, when a cystic NET is suspected, for which CEA levels are often low, chromogranin A and neuron-specific enolase measurements have evidenced some promise in determining the neuroendocrine nature of the cyst[43,44].
Mucins and cytokines analysis
Mucins (MUCs) are high-weight molecular glycoproteins that play an important role in the protection of the pancreatic duct lining[45]. Alterations in MUC glycosylation have been observed in malignant tissues. IPMNs express MUC2, MUCA5, and MUC4, whereas ductal adenocarcinoma expresses MUC1 but lacks MUC2[46].
An exploratory study by Lee et al. found that certain markers in cystic fluid, such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and hepatocyte growth factor (HGF), were present in significantly higher concentrations in inflammatory cysts than in BD-IPMNs[47].
Higher levels of interleukin L1β (IL1β) expression were associated with high-grade dysplasia or invasive carcinoma in IPMNs. IL1β distinguished high-risk cysts from low-risk cysts[48]. Other markers such as transforming growth factor β-1 (TGF-beta 1) and granulocyte colony-stimulating factor (G-CSF) are found in high concentrations in MD-IPMNs. TGF- beta 1 alone was found in MD-IPMNs but not in BD-IPMNs[49].
Molecular analysis
Mini-chromosome maintenance proteins (MCM 2e7) are expressed in a higher quantity by cancerous cells than by benign lesions. Their expression was identified in higher concentrations in pancreatic cancers and mucinous pancreatic cysts. Mini-chromosome maintenance 5 (MCM5) has a sensitivity of 73% and specificity of 50% in detecting cancer in pancreatic cysts[50].
MicroRNA expression is abundant in malignant pancreatic lesions. There have been attempts to use microRNA to stratify pancreatic lesions as malignant, premalignant, and benign[51,52]. A study that collected pancreatic fluid from 40 patients (yielding 14 IPMNs, 10 mucinous, 11 serous cystadenomas, and 5 benign cysts) found that miR-21, miR-221, and miR-17-3p were highly expressed in mucinous compared to non-mucinous cysts (P < 0.01). MiR-21 was found to have a specificity of 76% and a sensitivity of 80%[53].
Several miRNA panels were found to differentiate between benign and malignant cysts[54]. The SCA panel contained the following miRNAs: miR-31-5p, miR-483-5p, miR-99a-5p, and miR-375. It differentiated SCA from mucinous and pancreatic duct adenocarcinoma with 90% sensitivity and 100% specificity. The MCNs panel consisted of the following miRNAs: miR-10b 5p, miR-202-3p, miR-210, and miR-375. Its sensitivity and specificity were both 100%. The pancreatic duct adenocarcinoma panel contained miR-21-5p,
Global RNA gene expression profiling found that the neoplastic epithelium of mucinous cysts had 6.6 times more cathepsin E (CTSE) than a normal pancreatic ductal epithelium. This finding suggests that CTSE activity may be superior to other standard markers like CEA in identifying mucinous and malignant pancreatic cysts[55].
DNA analysis
DNA testing has emerged as a new adjunct tool in the assessment of PCLs. In a study of 11 malignant, 15 premalignant, and 10 benign cysts, K-Ras-2 mutation followed by an analysis of the loss of heterozygosity identified malignant cysts with a 91% sensitivity and a 93% specificity[33]. Mutations in KRAS were found in IPMNs and MCNs, whereas GNAS is frequently mutated IPMNs[56,57]. IPMNs with high-grade dysplasia or malignancy were found to have mutations in TP53, PIK3CA, PTEN and/or AKT1[58]. A study that analysed pancreatic fluid for genetic mutation using next-generation sequencing found that KRAS/GNAS mutations were highly sensitive for IPMNs and specific for mucinous cysts[58].
Through-the-needle micro-forceps biopsy
TTNB devices (Moray Micro-forceps, US Endoscopy, Mentor, Ohio, USA) have demonstrated their superiority over standard FNA with accuracy, sensitivity, and specificity of 78.8%, 82.2%, and 96.8%, respectively, in diagnosing pancreatic cyst subtypes[59]. Three passes on average have yielded adequate histology results. The TTNB offers an assessment of the cyst wall, which, when combined with the cytological and biochemical assessment of the aspirated fluid, improve diagnostic accuracy. The major drawback of this technique is that it only allows sampling of the posterior wall of the cyst from the point of entry of the needle. Therefore, the acquired tissue may not represent the actual dysplasia. The reported adverse events were bleeding (2%-4%) and pancreatitis (2%)[59,60].
Confocal laser endomicroscopy
nCLE is another novel technique that can be combined with EUS to enable imaging lesions at the subcellular level and to provide an optical biopsy[61,62]. It has been used in diagnosing lesions in different parts of the gastrointestinal tract. It involves passing a nCLE through a 19 G needle, which enables real-time microscopic visualisation of tissue. Pooled data produced a sensitivity of 82.4%, a specificity of 96.6%, and a diagnostic accuracy of 88.6% in diagnosing pancreatic cyst subtypes%[63]. The adverse events reported were mild bleeding, infection, and mild pancreatitis (1.4%-7%).
Pancreatoscopy
Per-oral pancreatoscopy (POP) has also been employed in the diagnostic work-up of MD-IPMNs or during intraoperative mapping[64]. It offers intraductal visualisation of the pancreatic duct. The reported malignant features in IPMNs were fish-egg-like projections with vascular images, villous types, and vegetative types[65]. The reported cannulation rate was 86%-100%. Although POP is reported to achieve technical success, its main disadvantage is the reported high rate of adverse events of 12%, with post-procedure pancreatitis at up to 10%[64]. Due to its high risk of associated pancreatitis, POP should be performed only after a diagnostic work-up using EUS and tissue sampling and after multidisciplinary discussion. Its role should be to help decide in equivocal cases, to assess the presence of malignant changes, and to guide the choice of the margin of resection of IPMNs[64].
Endoscopic retrograde cholangio-pancreatography
Endoscopic retrograde cholangio-pancreatography (ERCP) can help to confirm communication between a pancreatic cyst and the main pancreatic duct. The potential use of ERCP is to inform the differentiation of an MD-IPMN from chronic pancreatitis, but given the high risk of pancreatitis following ERCP, it is reserved for therapeutic purposes only[28,66].
CONCLUSION
Patients who have PCLs often undergo several tests to categorise their cysts and detect premalignant or malignant changes. Some of these tests can be invasive and pose the risk of developing complications. The assessment of PCLs often requires a combination of more than one test to achieve adequate diagnostic accuracy. The challenges in managing PCLs are mainly in differentiating mucinous from non-mucinous cysts and in detecting high-grade dysplasia or early cancer.
Attempts have been made to incorporate artificial intelligence (AI) technology into clinical practice to aid the management of PCLs and reduce human bias. Kurita et al. obtained a high diagnostic accuracy (92.9%) by constructing a diagnostic algorithm using relevant variables: CEA, CA 125, a cystic fluid amylase, the type of cyst, sex, cyst location, the connection of the pancreatic duct to the cyst, and the type of cyst[67]. This type of AI algorithm relies on the quality and availability of the relevant information that is fed into it. Its functionality may be impaired if information is missing. Kuwahara et al. obtained a diagnostic accuracy of 86.2% in detecting malignancy in IPMNs via deep learning using EUS images[68]. In addition, their study yielded higher accuracy than human diagnosis or the presence of mural nodules; however, there was more cancer compared to routine clinical practice. The study was limited by its sample size and retrospective nature. By using clinical, imaging, and molecular markers from 436 patients, another retrospective study developed a supervised learning software programme to categorise PCLs into those that require surgery or surveillance and those that can be safely discharged[69]. Using histopathology as a gold standard, the clinical management outcome from using the software was more accurate than from standard care.
EUS-guided pancreatic cyst ablation using ethanol and/or paclitaxel has been investigated for the non-surgical treatment of PCLs. It can provide a minimally invasive treatment, especially for those deemed at high risk for surgical resection. A small study (n = 13) reported complete or partial resolution in 12 patients[70]. A larger study (n = 162) confirmed its high efficacy in unilocular and small cysts with complete or partial resolution in 91.8%. During the follow-up period, out of 114 patients (post-ablation), only two had cyst recurrences in six years[71].
PCLs have stimulated considerable research on developing new imaging, endoscopic, and sampling techniques. The approach to each patient should be individualised based on their clinical status, the presence of co-morbidities, and the risk of malignancy. MRI may help to identify mucinous cysts or malignant changes. However, a combination of imaging morphology with EUS-FNA may be required. Cystic fluid biomarkers can be used as an adjunct in predicting high-risk PCLs and those requiring early surgical resections. TTNB, nCLE, and pancreatoscopy are promising new tests that can be used in the assessment and management of PCLs. Pancreatoscopy should be reserved for equivocal cases due to the high risk of pancreatitis. Some of the advances reported in this review may not be applicable in clinical practice due to their limited availability. Therefore, the classic high-risk physical symptoms remain the most important and valuable predictors of surgical therapy.
Future research is required to develop non-invasive tests and markers that can differentiate benign from premalignant or malignant PCLs.
DECLARATIONS
Authors’ contributionsMade substantial contributions to the conception and design of the article and interpreting the relevant literature: Jalal M, Gbadegesin S, Ibrahim S, Tehami N
Drafted the article or revised it critically for important intellectual content: Jalal M, Gbadegesin S, Ibrahim S, Tehami N
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Zerboni G, Signoretti M, Crippa S, Falconi M, Arcidiacono PG, Capurso G. Systematic review and meta-analysis: prevalence of incidentally detected pancreatic cystic lesions in asymptomatic individuals. Pancreatology 2019;19:2-9.
2. Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol 2019;10:10-27.
3. Munigala S, Gelrud A, Agarwal B. Risk of pancreatic cancer in patients with pancreatic cyst. Gastrointest Endosc 2016;84:81-6.
4. Schweber AB, Agarunov E, Brooks C, Hur C, Gonda TA. Prevalence, incidence, and risk of progression of asymptomatic pancreatic cysts in large sample real-world data. Pancreas 2021;50:1287-92.
5. Anand N, Sampath K, Wu BU. Cyst features and risk of malignancy in intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Clin Gastroenterol Hepatol 2013;11:913-21; quiz e59.
6. Okasha HH, Awad A, El-Meligui A, et al. Cystic pancreatic lesions, the endless dilemma. World J Gastroenterol 2021;27:2664-80.
7. Miller FH, Lopes Vendrami C, Recht HS, et al. Pancreatic cystic lesions and malignancy: assessment, guidelines, and the field defect. Radiographics 2022;42:87-105.
8. Buerke B, Domagk D, Heindel W, Wessling J. Diagnostic and radiological management of cystic pancreatic lesions: important features for radiologists. Clin Radiol 2012;67:727-37.
9. Scholten L, van Huijgevoort NCM, van Hooft JE, Besselink MG, Del Chiaro M. Pancreatic cystic neoplasms: different types, different management, new guidelines. Visc Med 2018;34:173-7.
10. Xu MM, Yin S, Siddiqui AA, et al. Comparison of the diagnostic accuracy of three current guidelines for the evaluation of asymptomatic pancreatic cystic neoplasms. Medicine 2017;96:e7900.
11. Miura T, Igarashi Y, Okano N, Miki K, Okubo Y. Endoscopic diagnosis of intraductal papillary-mucinous neoplasm of the pancreas by means of peroral pancreatoscopy using a small-diameter videoscope and narrow-band imaging. Dig Endosc 2010;22:119-23.
12. Cooper CL, O’Toole SA, Kench JG. Classification, morphology and molecular pathology of premalignant lesions of the pancreas. Pathology 2013;45:286-304.
13. Mukai H, Yasuda K, Nakajima M. Differential diagnosis of mucin-producing tumors of the pancreas by intraductal ultrasonography and peroral pancreatoscopy. Endoscopy 1998;30 Suppl 1:A99-102.
14. Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018;67:789-804.
15. Hisada Y, Nagata N, Imbe K, et al. Natural history of intraductal papillary mucinous neoplasm and non-neoplastic cyst: long-term imaging follow-up study. J Hepatobiliary Pancreat Sci 2017;24:401-8.
16. Lawson R, Savides T, Kwong W, Hunt G, Giap A. Risk of pancreatic cancer in patients with cystic lesions of the pancreas based upon EUS morphology. In Presented at 78th Annual Scientific Meeting of The American College of Gastroenterology; 11-16 October 2013. San Diego, CA, USA. Available from: https://journals.lww.com/ajg/Fulltext/2013/10001/Risk_of_Pancreatic_Cancer_in_Patients_with_Cystic.305.aspx [Last accessed on 15 Apr 2023].
17. Lee SH, Park JK, Woo SM, et al. Natural history of branch-duct type intraductal papillary mucinous neoplasms of the pancreas. Korean J Gastroenterol 2007;49:24-30. Available from: https://europepmc.org/article/med/18167430 [Last accessed on 15 Apr 2023]
18. Gonzalez I, Tang RS, Munroe CA, et al. Natural history of pancreatic cysts < 6 cm with benign EUS appearance without surgical resection. In Presented at Digestive Disease Week; 19-22 May 2012; San Diego, CA, USA. Available from: https://doi.org/10.1016/j.gie.2012.04.370 [Last accessed on 15 Apr 2023].
19. Nougaret S, Reinhold C, Chong J, et al. Incidental pancreatic cysts: natural history and diagnostic accuracy of a limited serial pancreatic cyst MRI protocol. Eur Radiol 2014;24:1020-9.
20. Lekkerkerker SJ, Fockens P, Besselink M, Rauws E, Van Hooft JE. 105 malignant progression during long-term follow-up of pancreatic cysts: how often do we change teatment strategy? Gastrointestinal Endoscopy 2015;81:AB115.
21. Broughton J, Lipschitz J, Cantor M, Moffatt D, Abdoh A, McKay A. Determining the natural history of pancreatic cystic neoplasms: a Manitoban cohort study. HPB 2016;18:383-8.
22. Pak LM, D’Angelica MI, DeMatteo RP, et al. Natural history of patients followed radiographically with mucinous cysts of the pancreas. J Gastrointest Surg 2017;21:1599-605.
23. Ohno E, Hirooka Y, Kawashima H, et al. Natural history of pancreatic cystic lesions: a multicenter prospective observational study for evaluating the risk of pancreatic cancer. J Gastroenterol Hepatol 2018;33:320-8.
24. Lee BS, Nguyen AK, Tekeste TF, et al. Long-term follow-up of branch-duct intraductal papillary mucinous neoplasms with No change in first 5 years of diagnosis. Pancreatology 2021;21:144-54.
25. Farrell JJ. Prevalence, diagnosis and management of pancreatic cystic neoplasms: current status and future directions. Gut Liver 2015;9:571-89.
26. Zhang XP, Yu ZX, Zhao YP, Dai MH. Current perspectives on pancreatic serous cystic neoplasms: diagnosis, management and beyond. World J Gastrointest Surg 2016;8:202-11.
27. Mohamed E, Jackson R, Halloran CM, Ghaneh P. Role of radiological imaging in the diagnosis and characterization of pancreatic cystic lesions: a systematic review. Pancreas 2018;47:1055-64.
28. Jabłońska B, Szmigiel P, Mrowiec S. Pancreatic intraductal papillary mucinous neoplasms: current diagnosis and management. World J Gastrointest Oncol 2021;13:1880-95.
29. Gavini H, Lee JH. Endoscopic ultrasound-guided endotherapy. J Clin Gastroenterol 2015;49:185-93.
30. Kawada N, Uehara H, Katayama K, et al. Diagnostic clues and subsequent examinations that detected small pancreatic cancer. Hepatogastroenterology 2012;59:1665-9.
31. Serrani M, Lisotti A, Caletti G, Fusaroli P. Role of contrast harmonic-endoscopic ultrasound in pancreatic cystic lesions. Endosc Ultrasound 2017;6:25-30.
32. Muthusamy VR, Chandrasekhara V, Acosta RD, et al. The role of endoscopy in the diagnosis and treatment of cystic pancreatic neoplasms. Gastrointest Endosc 2016;84:1-9.
33. Boot C. A review of pancreatic cyst fluid analysis in the differential diagnosis of pancreatic cyst lesions. Ann Clin Biochem 2014;51:151-66.
34. Kadiyala V, Lee LS. Endosonography in the diagnosis and management of pancreatic cysts. World J Gastrointest Endosc 2015;7:213-23.
35. Thosani N, Thosani S, Qiao W, Fleming JB, Bhutani MS, Guha S. Role of EUS-FNA-based cytology in the diagnosis of mucinous pancreatic cystic lesions: a systematic review and meta-analysis. Dig Dis Sci 2010;55:2756-66.
36. Thornton GD, McPhail MJ, Nayagam S, Hewitt MJ, Vlavianos P, Monahan KJ. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: a meta-analysis. Pancreatology 2013;13:48-57.
37. Soyer OM, Baran B, Ormeci AC, et al. Role of biochemistry and cytological analysis of cyst fluid for the differential diagnosis of pancreatic cysts: a retrospective cohort study. Medicine 2017;96:e5513.
38. Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology 2004;126:1330-6.
39. Cao S, Hu Y, Gao X, Liao Q, Zhao Y. Serum carbohydrate antigen 19-9 in differential diagnosis of benign and malignant pancreatic cystic neoplasms: a meta-analysis. PLoS One 2016;11:e0166406.
41. Khan I, Baig M, Bandepalle T, Puli SR. Utility of cyst fluid carcinoembryonic antigen in differentiating mucinous and non-mucinous pancreatic cysts: an updated meta-analysis. Dig Dis Sci 2022;67:4541-8.
42. Smith ZL, Satyavada S, Simons-Linares R, et al. Intracystic glucose and carcinoembryonic antigen in differentiating histologically confirmed pancreatic mucinous neoplastic cysts. Am J Gastroenterol 2022;117:478-85.
43. Faias S, Prazeres S, Cunha M, et al. Chromogranin a and NSE in cystic pancreatic neuroendocrine tumors. Clin Res Hepatol Gastroenterol 2021;45:101601.
44. Levy A, Popovici T, Bories PN. Tumor markers in pancreatic cystic fluids for diagnosis of malignant cysts. Int J Biol Markers 2017;32:e291-6.
45. Hollingsworth MA, Strawhecker JM, Caffrey TC, Mack DR. Expression of MUC1, MUC2, MUC3 and MUC4 mucin mRNAs in human pancreatic and intestinal tumor cell lines. Int J Cancer 1994;57:198-203.
46. Maker AV, Carrara S, Jamieson NB, et al. Cyst fluid biomarkers for intraductal papillary mucinous neoplasms of the pancreas: a critical review from the international expert meeting on pancreatic branch-duct-intraductal papillary mucinous neoplasms. J Am Coll Surg 2015;220:243-53.
47. Lee LS, Banks PA, Bellizzi AM, et al. Inflammatory protein profiling of pancreatic cyst fluid using EUS-FNA in tandem with cytokine microarray differentiates between branch duct IPMN and inflammatory cysts. J Immunol Methods 2012;382:142-9.
48. Maker AV, Katabi N, Qin LX, et al. Cyst fluid interleukin-1beta (IL1beta) levels predict the risk of carcinoma in intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res 2011;17:1502-8.
49. Lee LS, Bellizzi AM, Banks PA, et al. Differentiating branch duct and mixed IPMN in endoscopically collected pancreatic cyst fluid via cytokine analysis. Gastroenterol Res Pract 2012;2012:247309.
50. Keane MG, Huggett MT, Chapman MH, et al. Diagnosis of pancreaticobiliary malignancy by detection of minichromosome maintenance protein 5 in biliary brush cytology. Br J Cancer 2017;116:349-55.
51. Matthaei H, Wylie D, Lloyd MB, et al. miRNA biomarkers in cyst fluid augment the diagnosis and management of pancreatic cysts. Clin Cancer Res 2012;18:4713-24.
52. Farrell JJ, Toste P, Wu N, et al. Endoscopically acquired pancreatic cyst fluid microRNA 21 and 221 are associated with invasive cancer. Am J Gastroenterol 2013;108:1352-9.
53. Ryu JK, Matthaei H, Dal Molin M, et al. Elevated microRNA miR-21 levels in pancreatic cyst fluid are predictive of mucinous precursor lesions of ductal adenocarcinoma. Pancreatology 2011;11:343-50.
54. Lee LS, Szafranska-Schwarzbach AE, Wylie D, et al. Investigating MicroRNA expression profiles in pancreatic cystic neoplasms. Clin Transl Gastroenterol 2014;5:e47.
55. Pontious C, Kaul S, Hong M, et al. Cathepsin E expression and activity: role in the detection and treatment of pancreatic cancer. Pancreatology 2019;19:951-6.
56. Singhi AD, Nikiforova MN, Fasanella KE, et al. Preoperative GNAS and KRAS testing in the diagnosis of pancreatic mucinous cysts. Clin Cancer Res 2014;20:4381-9.
57. Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 2011;3:92ra66.
58. Singhi AD, McGrath K, Brand RE, et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut 2018;67:2131-41.
59. Facciorusso A, Del Prete V, Antonino M, Buccino VR, Wani S. Diagnostic yield of EUS-guided through-the-needle biopsy in pancreatic cysts: a meta-analysis. Gastrointest Endosc 2020;92:1-8.e3.
60. Yang D, Samarasena JB, Jamil LH, et al. Endoscopic ultrasound-guided through-the-needle microforceps biopsy in the evaluation of pancreatic cystic lesions: a multicenter study. Endosc Int Open 2018;6:E1423-30.
61. Guo J, Bhutani MS, Giovannini M, et al. Can endoscopic ultrasound-guided needle-based confocal laser endomicroscopy replace fine-needle aspiration for pancreatic and mediastinal diseases? Endosc Ultrasound 2017;6:376-81.
62. Kohoutova D, Zar S, Repak R, Vlavianos P, Bures J. Pancreatic cysts: diagnostic role of EUS-guided microforceps biopsy and confocal laser endomicroscopy. Gastroenterol Res Pract 2019;2019:3431048.
63. Facciorusso A, Buccino VR, Sacco R. Needle-based confocal laser endomicroscopy in pancreatic cysts: a meta-analysis. Eur J Gastroenterol Hepatol 2020;32:1084-90.
64. de Jong DM, Stassen PMC, Groot Koerkamp B, et al. The role of pancreatoscopy in the diagnostic work-up of intraductal papillary mucinous neoplasms: a systematic review and meta-analysis. Endoscopy 2023;55:25-35.
65. Hara T, Yamaguchi T, Ishihara T, et al. Diagnosis and patient management of intraductal papillary-mucinous tumor of the pancreas by using peroral pancreatoscopy and intraductal ultrasonography. Gastroenterology 2002;122:34-43.
66. Clores MJ, Thosani A, Buscaglia JM. Multidisciplinary diagnostic and therapeutic approaches to pancreatic cystic lesions. J Multidiscip Healthc 2014;7:81-91.
67. Kurita Y, Kuwahara T, Hara K, et al. Diagnostic ability of artificial intelligence using deep learning analysis of cyst fluid in differentiating malignant from benign pancreatic cystic lesions. Sci Rep 2019;9:6893.
68. Kuwahara T, Hara K, Mizuno N, et al. Usefulness of deep learning analysis for the diagnosis of malignancy in intraductal papillary mucinous neoplasms of the pancreas. Clin Transl Gastroenterol 2019;10:1-8.
69. Springer S, Masica DL, Dal Molin M, et al. A multimodality test to guide the management of patients with a pancreatic cyst. Sci Transl Med 2019:11.
70. Oh HC, Seo DW, Lee TY, et al. New treatment for cystic tumors of the pancreas: EUS-guided ethanol lavage with paclitaxel injection. Gastrointest Endosc 2008;67:636-42.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Jalal M, Gbadegesin SA, Ibrahim S, Tehami N. Recent advances in detecting premalignant pancreatic cysts . J Cancer Metastasis Treat 2023;9:11. http://dx.doi.org/10.20517/2394-4722.2022.122
AMA Style
Jalal M, Gbadegesin SA, Ibrahim S, Tehami N. Recent advances in detecting premalignant pancreatic cysts . Journal of Cancer Metastasis and Treatment. 2023; 9: 11. http://dx.doi.org/10.20517/2394-4722.2022.122
Chicago/Turabian Style
Jalal, Mustafa, Sebastine A. Gbadegesin, Sazgar Ibrahim, Nadeem Tehami. 2023. "Recent advances in detecting premalignant pancreatic cysts " Journal of Cancer Metastasis and Treatment. 9: 11. http://dx.doi.org/10.20517/2394-4722.2022.122
ACS Style
Jalal, M.; Gbadegesin SA.; Ibrahim S.; Tehami N. Recent advances in detecting premalignant pancreatic cysts . J. Cancer. Metastasis. Treat. 2023, 9, 11. http://dx.doi.org/10.20517/2394-4722.2022.122
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 4 clicks
Cite This Article 4 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.