Bio-fabrication of liver cancer stem cells and testing of gemcitabine
Abstract
Aim: Cancer stem cells are cell populations that are essential in drug resistance and cancer metastasis. Some liver cancer cells exhibit the characteristics of cancer stem cells, and it is crucial to study the activities and interactions of drugs in these cells. Huh7 is a human liver cancer cell with stem cell biomarkers and is used with induced pluripotent stem cells to form various cancer organoids through encapsulation methods. Due to their ease of use without animal testing, bio-fabrication studies of cell-encapsulated models have gained importance in the pharmaceutical industry in recent years. This study aimed to biofabricate Huh7 human liver cancer stem cells by alginate encapsulation and test gemcitabine’s efficacy.
Methods: Huh7 cells were encapsulated with alginate (0.8% w/v) and fibronectin, and their viability was evaluated with 3.2 µM gemcitabine on days 1, 3, 6, 9, and 12. Furthermore, gene expressions of stem cell markers CD90 and AFP were evaluated in encapsulated Huh7 cells by qPCR. In addition, IL-6 secretion in the cell medium was measured by ELISA for the tumor microenvironment.
Results: Encapsulation of Huh7 cells was found to maintain their viability and stem cell properties for up to 12 days. In addition, alginate-encapsulated Huh7 cells were bio-fabricated to demonstrate long-term gemcitabine response. While the effect of the gemcitabine was evaluated in alginate-encapsulated Huh7 cells, CD90 and AFP mRNA levels were significantly reduced in the cells and IL-6 secretion was decreased in the tumor microenvironment.
Conclusion: This study demonstrated that bio-fabrication of alginate-encapsulated Huh7 cells is a novel approach for long-term drug testing in liver cancer models. Bio-fabricated alginate-encapsulated cancer stem cells may be a cheaper and faster method for the testing of many drugs.
Keywords
INTRODUCTION
Liver cancer is the most common type of cancer and ranks second in metastasis and death rates. It is known that such a common type of cancer involves complex processes at both the pathological and molecular levels. Although liver cancer cells originate from many different origins with heterogeneity, they are cancer stem cells that provide continuous self-renewal and recurrence of these cells. Cancer stem cells have self-renewal and pluripotency properties and arise from the transformation of progenitor cells. Many cancer stem cells of different origin that provide this heterogeneity is responsible for tumor development and control metastasis[1]. Many liver cancer cells carry these characteristics of cancer stem cells and are involved in the complex process at the molecular level. It is also known that cancer stem cells reduce the effectiveness of treatments and contribute to the recurrence of the disease by becoming more drug-resistant[2]. Liver cancer stem cells contain typical stem cell markers (CD90, CD133, CD44, Nanog, Sox-2, Oct-4, etc.). In addition, they also contain liver cancer-specific markers (AFP, TGF-β, EpCAM, etc.). Liver cancer stem cells can arise from intrahepatic tissues in the liver and bile duct and extrahepatic from bone marrow or peripheral blood cells. The morphological structures of these cells and the surface markers they carry differ[3].
Bio-fabrication is the production of biological materials without the need for a living organism. In this process, an external matrix is used to create organoids in a three-dimensional structure and to produce biological structures such as tumors and muscle tissue[4]. In recent years, bio-fabrication has contributed to the development of interdisciplinary studies in many studies of animal-free drug testing and toxicity testing. However, 3D bioprinting research in bio-fabrication is quite expensive because it requires hardware and software, and it is not used for drug toxicity testing because it provides non-sterile conditions[5]. Primarily, studies on 3D bioprinters are in tissue engineering and regenerative medicine. Hydrogels, biopolymers, gelatins, and nanomaterials are mainly used in drug research[6]. In order to measure the behavior of drugs, it is necessary to mimic the micro and nano environments of biological structures in sterile environments. Research with nano-biomaterials has progressed to the point where even systems that can enter the cell and deliver drugs to the target area have been developed[7]. In this study, the response to gemcitabine was tested by placing liver cancer stem cells in alginate hydrogels, and their potential to be used for drug toxicity testing in a bio-fabrication model was investigated.
METHODS
Cells and culture conditions
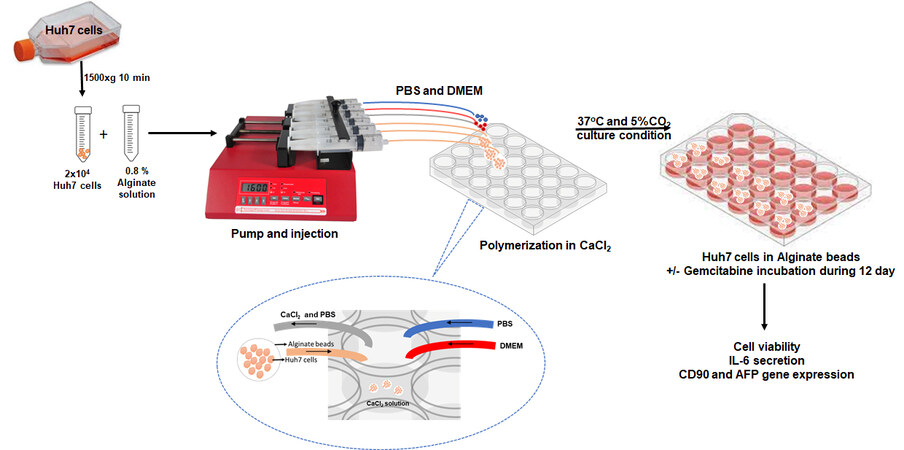
Huh7 human liver cancer stem cells were grown in T-75 cell culture flasks in DMEM medium (containing 10% FBS, 1% penicillin-streptomycin, 4 mM glutamine, 1 mM sodium pyruvate) at 37 °C and 5% CO2. After a sufficient level of proliferation had been achieved in the Huh7 cells, the confluent cells were harvested using trypsin-EDTA. Huh7 cells were washed with DPBS and counted. After the preparation of the cells, the cells were used in alginate capsules for bio-fabrication studies. The design of the study is given in Figure 1.
Figure 1. Experimental design (2 × 104 Huh7 cells and alginate solution were mixed and placed in the microfluidic syringe pump. The microfluidic syringe pump was used to inject the mixture at a flow rate of 20 μL/min into a 24-well plate. After polymerizing the alginate with 0.1 M CaCl2, the beads were washed with PBS and cultured using DMEM medium. The cell viability, IL-6 secretion, CD90, and AFP levels of the Huh7 cells encapsulated in alginate were examined for 12 days with/without gemcitabine).
MTT assay
MTT test was performed to determine the gemcitabine IC50 value. Cells were seeded in 1 × 104 numbers in a volume of 200 µL on 96 well plates. Gemcitabine was incubated at concentrations of 0.1-1,000 µM for 24 h. After incubation, the cell medium was discarded, and a fresh DMEM medium was added. Next, 5 mM MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution (ODC Research and Development Inc) was added to the cells and incubated for 4 h. After incubation, 10 µL of DMSO was added, and the formazone crystals were dissolved. Absorbance values were read at 540 nm in an ELISA cover. The experiments were run in triplicate, and the IC50 value was calculated for gemcitabine.
Bio-fabrication studies with alginate capsulated Huh7 cell
Sodium alginate powder was dissolved in Ca-free and Mg-free Dulbecco’s phosphate-buffered saline (DPBS, Gibco 14190144) buffer, and its final concentration was adjusted to 0.8% w/v[8]. Then, 2 × 104 Huh7 cells were placed in 10 µL fibronectin (0.5 mg/mL, Sigma F0556 MO, USA) and mixed with 1 mL of alginate solution (0.8% w/v). A 20-gauge syringe tip was inserted into the injectors using a microfluidic syringe pump (NE-1600, Rotalab). Alginate and cell mixture were injected into 24-well plates at a flow rate of
Cell viability of alginate capsulated cells
The viability of Huh7 cells in alginate hydrogel was measured by Calcein staining[9]. Encapsulated or encapsulated cells treated with gemcitabine (3.2 µM) were stained with calcein on days 1, 3, 6, 9, and 12. After incubation, the cells were incubated at 37 °C for 20 min with buffer containing (94 mM NaCI,
CD90 and AFP gene expression
Whether Huh7 cells in alginate hydrogel contained cancer stem cell markers was checked by CD90 and AFP gene expression analysis. Huh7 cells were collected on days 1, 3, 6, 9, and 12 from alginate encapsulation. Cells were solubilized from Alginate first. For solubilization, buffer containing 94 mM NaCl, 350 mM Na Citrate, 35 mM MOPS was used and incubated at 37 °C for 20 min. Then it was centrifuged at 1,500× g at
IL-6 assay
Secretion of IL-6, a cytokine for measuring the tumor microenvironment, was performed with the aid of the ELISA kit according to the manufacturer’s protocol (ODC0003eh, ODC Inc). Cell media were collected on days 1st, 3rd, 6th, 9th, and 12th from Huh7 cells encapsulated with alginate and Huh7 cells treated with gemcitabine (3.2 µM). First, the medium was centrifuged at 1,500× g for 10 min, the upper phase was separated, and IL-6 secretion was examined. Then, 100 µL of supernatant was loaded into the wells in the plate and incubated at 37 °C for 2 h. After washing three times with wash buffer, they were treated with HRP-conjugated secondary antibody. Then, washing was done three times again, and the color changes of 100 µL of 3,3′,5,5′-Tetramethylbenzidine solution were measured at 450 nm in an ELISA reader (Biotek, EPOCH).
RESULTS
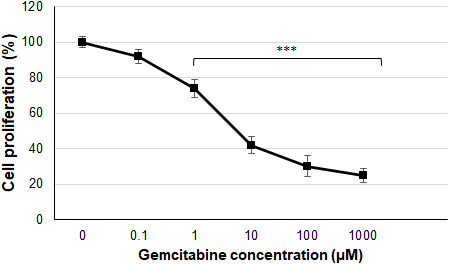
The effect of gemcitabine on Huh7 cells was first determined and cells were incubated for 24 h to make dose adjustments (0.1-1,000 µM). After MTT experiments in cells, the IC50 value of gemcitabine was found to be 3.2 ± 0.8 µM for Huh7 cells. Gemcitabine significantly reduced Huh7 cell proliferation at 1 µM, 10 µM,
Figure 2. Cell proliferation levels of Huh7 cells with gemcitabine (***P < 0.001 compared to control cells).
The radius of the alginate capsules was measured and checked under the microscope to determine whether they contained cells. Alginate capsules are 770 + 82 mm in diameter, and the viscosity was determined as 552 mPa s at 25 °C [Figure 3A].
Figure 3. (A) Huh7 cells (top-2D) and Huh7 alginate beads (bottom-3D) morphological image. (B) Cell viability levels of Huh7 cells in alginate beads (+++,***P < 0.001, ++P < 0.01. +compared to without gemcitabine treated Huh7 cells, *compared to with gemcitabine treated Huh7 cells).
Alginate encapsulated Huh7 cells, and gemcitabine (3.2 µM) application determined cell viability with calcein depending on the days. It is observed that Huh7 cells preserved their viability in alginate beads until the 12th day [Figure 3B]. When gemcitabine was applied, Huh7 cells in alginate beads were compared with Huh7 cells that were not treated with gemcitabine, and cell viability was found to decrease significantly from day 1 to day 12 (P < 0.01, P < 0.001). When the cells treated with gemcitabine were compared among themselves, it was observed that cell viability decreased from day 3 until day 12 compared to Huh7 cells without alginate encapsulation (P < 0.001).
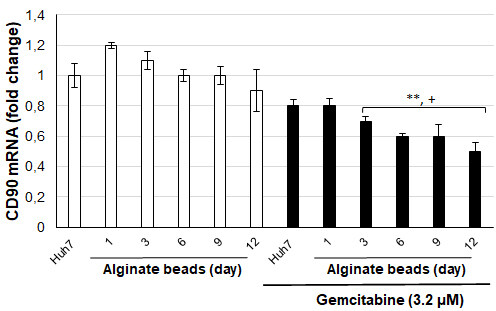
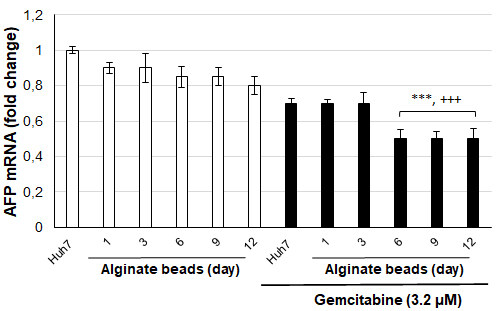
The gene expression levels of CD90 and AFP, which are stem cell markers, were tested depending on the days following the application of alginate-encapsulated Huh7 cells and gemcitabine (3.2 µM) [Figure 4]. CD90 mRNA levels decreased slightly from day 6 in alginate-encapsulated Huh7 cells, but this decrease was insignificant. However, CD90 mRNA levels were significantly decreased in alginate-encapsulated Huh7 cells treated with gemcitabine from day 3 compared to control cells (P < 0.05, P < 0.01). AFP mRNA levels were not significantly different in alginate-encapsulated Huh7 cells, similar to CD90 mRNA levels [Figure 5]. However, AFP mRNA levels in alginate-encapsulated Huh7 cells treated with gemcitabine were significantly reduced from day 6 compared to with/without gemcitabine-treated Huh7 cells (P < 0.001). Huh7 cells are adherent cells. A slight decrease in AFP and CD90 mRNA levels in control cells after 6 days results from their inability to adhere and proliferate. The cells maintain their vitality through colony formation, but there is a slowdown in cell division. When Huh7 cells that remained viable in alginate beads were exposed to gemcitabine, changes in their cellular responses were measured at mRNA levels and found to be significant.
Figure 4. CD90 mRNA levels of Huh7 cells in alginate beads (+P < 0.05, **P < 0.01. +compared to without gemcitabine treated Huh7 cells,
Figure 5. AFP mRNA levels of Huh7 cells in alginate beads (+++,***P < 0.001. +compared to without gemcitabine treated Huh7 cells,
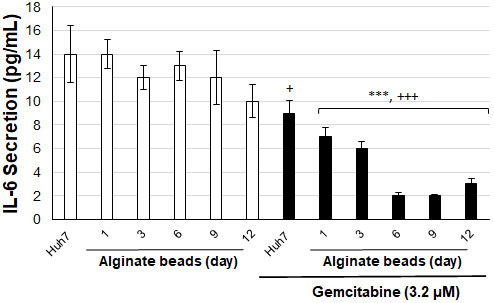
To evaluate the tumor microenvironment in cells, IL-6 secretion levels were tested in alginate-encapsulated Huh7 cells and cell medium depending on the days of gemcitabine (3.2 µM) application [Figure 6]. There was no significant change in IL-6 secretion levels of Huh7 cells in alginate beads until day 12. When gemcitabine was applied, Huh7 cells in alginate beads were compared with Huh7 cells that were not treated with gemcitabine, and IL-6 secretion levels were significantly reduced from day 1 to day 12
DISCUSSION
It is essential to detect drug and drug interactions for liver cancer. Cell culture studies are common on overcoming drug resistance created by liver stem cells and their drug interactions. However, there is a great need for systems produced by bio-fabrication so that these studies are within a standard and the answers can be studied with multi drugs.
Gemcitabine (2-2-difluoro deoxycytidine) is a pyrimidine analog used in cancer treatment. Gemcitabine enters the cell, inhibits DNA synthesis, and stops the proliferation of cancer cells. Approved by the FDA in the United States, this drug can be used alone or in combination with other antineoplastic agents to treat various cancers (liver, ovary, bladder, lung, breast, etc.)[10]. We modeled whether cellular changes could be detected when gemcitabine was administered to Huh7 cells encapsulated with bio-fabricated alginate. We tested whether cellular responses with/without gemcitabine changed over 12 days.
Alginate is a block copolymer consisting of mannuronic and guluronic acid and is often used in biological materials[11]. Alginates, rich in guluronic acid in cell applications, are the most suitable for encapsulation due to their viscosity[12,13]. In studies on bio-fabrication using alginate, 3-dimensional porous scaffolds were made from 2% w/v alginate using NIH/3T3 mouse fibroblast cells. It has been tested that the cells can survive in this bioink within two weeks[14]. Encapsulation of stem cells is very important in encapsulation studies with alginate and we have succeeded in encapsulating umbilical cord mesenchymal stem cells in this regard[8]. It has been shown that TNF-alpha, a proinflammatory cytokine, is secreted and biocompatible in monocyte encapsulation by cross-linking alginate with poly-L-lysine[15]. In a study using human pancreatic cancer cell lines (KP1N cells), it was reported that the vitality was preserved in hydrogels infused with alginate, and positive results were obtained in drug treatment experiments, and it was published as a protocol. The researchers emphasized that with hand-made alginate hydrogels, studies are limited if both the optimization and the number of cells are not stable, so there is a need for fabrication systems that contain the same proportion of cells with a large number of beads[16]. It has been shown that 3D structures containing polysaccharide alginate, periostin, and hydroxyapatite using MCF7 and MDA-MB-231 human breast cancers are more effective in drug trials than conventional cell cultures. In particular, it has been reported that these systems preserve the characteristics of cancer stem cells and can produce epithelial-mesenchymal transition, differentiation, and proliferation markers at both gene and protein levels[17]. In a study conducted in the development of in vitro drug screening tissue model systems, a platform was developed by co-culturing MCF-7 breast cancer cell lines and HEPG-2 liver cells. In the study, HepG2 cells were encapsulated in alginate hydrogels, and MCF7 cells were placed on a porous disc. They emphasized that hybrid systems were screened better than standard cell cultures when hepatotoxicity tests were performed by applying coumarin-based drugs for three days for drug transformation[18]. Cervical cancer 3D models made with increasing alginate concentrations have been shown to ensure the continuity of EMT and stem cell factors in the medium[19]. Our study showed that the cancer stem cell biomarkers CD90 and AFP were maintained for 12 days in alginate encapsulated cells. The researchers reported that critical determinants of tumor angiogenesis and neovascularisation of the microenvironment in tumor cells had been identified using alginate-encapsulated cells in hypoxia studies. In the study, cells encapsulated in a hypoxic environment were used to measure levels of interleukin-8 (IL-8). They found that 3D models are more suitable for studying complex physical and chemical processes in the neovascularization and angiogenic processes of the cancer microenvironment[20]. Our study examined the levels of IL-6, a proinflammatory cytokine, to monitor the microenvironment. We showed that alginate-encapsulated Huh7 cells secrete IL-6 for 12 days and that this model can be used to study the microenvironment for drug interactions in cancer. In another study, the effect of imatinib mesylate was investigated and the extracellular microenvironment was used to evaluate the biological and immunological properties of 3D alginate-based hydrogels prepared with neuroblastoma cells. The study reported that 3D alginate-based hydrogels could be effective in testing the efficacy of personalized therapeutic approaches for treating neuroblastoma and that they could be used in a systematic and reliable manner[21].
In Conclusion, The alginate encapsulation technique is mainly used for drug release or cell storage studies. There has been limited research on a bio-fabrication method for drug testing. Through long-term preservation of liver cancer stem cell properties, this study has shown that they can be used for drug interaction and metabolism studies. This research has highlighted the need to develop systems that will make it cheaper and faster to test drugs using liver cancer stem cells without the use of laboratory animals, using the bio-fabrication method. The advantage of this is that many drugs can be tested at the same time. The advantage is that many drugs can be tested together, but the disadvantage is that different types of cells cannot be in use together. For use in preclinical testing platforms, the use of different cell types and co-culture conditions should be expanded through bio-fabrication research.
DECLARATIONS
AcknowledgmentsWe thank ODC Research and Development Inc for their support in materials.
Authors’ contributionsMade substantial contributions to the conception and design of the study and performed data analysis and interpretation: Cevik E, Erdogan O, Cevik O
Performed data acquisition and provided administrative, technical, and material support: Cevik E, Cevik O
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported by Aydin Adnan Menderes University Research Grant (ADU-TPF-21043).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateEthics committee approval is not required because Huh7 cells are commercially available.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Afify SM, Sanchez Calle A, Hassan G, et al. A novel model of liver cancer stem cells developed from induced pluripotent stem cells. Br J Cancer 2020;122:1378-90.
2. Liu YM, Li XF, Liu H, Wu XL. Ultrasound-targeted microbubble destruction-mediated downregulation of CD133 inhibits epithelial-mesenchymal transition, stemness and migratory ability of liver cancer stem cells. Oncol Rep 2015;34:2977-86.
3. Castelli G, Pelosi E, Testa U. Liver cancer: molecular characterization, clonal evolution and cancer stem cells. Cancers 2017;9:127.
4. Marzio N, Eglin D, Serra T, Moroni L. Bio-fabrication: convergence of 3D bioprinting and nano-biomaterials in tissue engineering and regenerative medicine. Front Bioeng Biotechnol 2020;8:326.
5. Kahl M, Gertig M, Hoyer P, Friedrich O, Gilbert DF. Ultra-low-cost 3D bioprinting: modification and application of an off-the-shelf desktop 3d-printer for biofabrication. Front Bioeng Biotechnol 2019;7:184.
6. Moroni L, Boland T, Burdick JA, et al. Biofabrication: a guide to technology and terminology. Trends Biotechnol 2018;36:384-402.
7. Cenik M, Abas BI, Kocabiyik B, Demirbolat GM, Cevik O. Development of a new drug delivery system from hela-derived exosomes and the effect of docetaxel-loaded exosomes on mitochondrial apoptosis. J Pharm Innov 2021;17:931-9.
8. Gumus E, Abas BI, Cevik E, Kocabiyik B, Cenik M, Cevik O. Alginate encapsulation induce colony formation with umbilical cord-derived mesenchymal stem cells. Biomed Res 2021;4:113-21.
9. Galateanu B, Dimonie D, Vasile E, Nae S, Cimpean A, Costache M. Layer-shaped alginate hydrogels enhance the biological performance of human adipose-derived stem cells. BMC Biotechnol 2012;12:35.
10. Chen Z, Xie H, Hu M, Huang T, Hu Y, Sang N, Zhao Y. Recent progress in treatment of hepatocellular carcinoma. Am J Cancer Res 2020;10:2993.
11. Vos P, Lazarjani HA, Poncelet D, Faas MM. Polymers in cell encapsulation from an enveloped cell perspective. Adv Drug Deliv Rev 2014;67-68:15-34.
12. Bhujbal SV, Paredes-Juarez GA, Niclou SP, de Vos P. Factors influencing the mechanical stability of alginate beads applicable for immunoisolation of mammalian cells. J Mech Behav Biomed Mater 2014;37:196-208.
13. Kummerfeld G, Nair A, Ko S, et al. Alginate composition, temperature, and presence of islet tissue influence microcapsule permeability. In: 10th World Biomaterials Congress, Montréal, Canada, 17-22 May 2016.
14. Hazur J, Detsch R, Karakaya E, et al. Improving alginate printability for biofabrication: establishment of a universal and homogeneous pre-crosslinking technique. Biofabrication 2020;12:045004.
15. Hajifathaliha F, Mahboubi A, Nematollahi L, Mohit E, Bolourchian N. Comparison of different cationic polymers efficacy in fabrication of alginate multilayer microcapsules. Asian J Pharm Sci 2020;15:95-103.
16. Davoudi F, Ghorbanpoor S, Yoda S, et al. Alginate-based 3D cancer cell culture for therapeutic response modeling. STAR Protoc 2021;2:100391.
17. Svanström A, Rosendahl J, Salerno S, et al. Optimized alginate-based 3D printed scaffolds as a model of patient derived breast cancer microenvironments in drug discovery. Biomed Mater 2021;16:045046.
18. Lan SF, Starly B. Alginate based 3D hydrogels as an in vitro co-culture model platform for the toxicity screening of new chemical entities. Toxicol Appl Pharmacol 2011;256:62-72.
19. Pang Y, Mao SS, Yao R, et al. TGF-β induced epithelial-mesenchymal transition in an advanced cervical tumor model by 3D printing. Biofabrication 2018;10:044102.
20. DelNero P, Lane M, Verbridge SS, et al. 3D culture broadly regulates tumor cell hypoxia response and angiogenesis via pro-inflammatory pathways. Biomaterials 2015;55:110-8.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Cevik E, Erdogan O, Cevik O. Bio-fabrication of liver cancer stem cells and testing of gemcitabine. J Cancer Metastasis Treat 2023;9:15. http://dx.doi.org/10.20517/2394-4722.2022.114
AMA Style
Cevik E, Erdogan O, Cevik O. Bio-fabrication of liver cancer stem cells and testing of gemcitabine. Journal of Cancer Metastasis and Treatment. 2023; 9: 15. http://dx.doi.org/10.20517/2394-4722.2022.114
Chicago/Turabian Style
Cevik, Evrim, Omer Erdogan, Ozge Cevik. 2023. "Bio-fabrication of liver cancer stem cells and testing of gemcitabine" Journal of Cancer Metastasis and Treatment. 9: 15. http://dx.doi.org/10.20517/2394-4722.2022.114
ACS Style
Cevik, E.; Erdogan O.; Cevik O. Bio-fabrication of liver cancer stem cells and testing of gemcitabine. J. Cancer. Metastasis. Treat. 2023, 9, 15. http://dx.doi.org/10.20517/2394-4722.2022.114
About This Article
Copyright
Data & Comments
Data

 Cite This Article 3 clicks
Cite This Article 3 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.