Development and validation of a survival nomogram for muscle-invasive bladder cancer in the elderly: using competing risk models and propensity matching to apply the prediction tool
Abstract
Aim: Patients with muscle-invasive bladder cancer (MIBC) have a low survival rate, with a 5-year survival of approximately 45%, regardless of the treatment received. The risk of death within 5 years after radical cystectomy in patients with MIBC remains as high as 60%. Over 80% of patients with bladder cancer are over 65. Therefore, identifying prognostic correlates associated with radical cystectomy in older patients with MIBC could improve survival rates. In addition, radiotherapy and chemotherapy are particularly important as adjuvant treatments for MIBC patients undergoing radical cystectomy. Therefore, this study aimed to find risk factors for cancer-specific survival (CSS) and overall survival (OS) after radical cystectomy in elderly MIBC patients. The difference in survival between radiotherapy and chemotherapy was analyzed by Kaplan-Meier (K-M) curves to provide theoretical support for whether radiotherapy is recommended for such patients.
Methods: Patients 65 or older diagnosed with MIBC with radical cystectomy between 2004-2018 were obtained from the Surveillance, Epidemiology, and End Results (SEER) database. 2004-2015 patients were subjected to column line plot production and internal validation, and 2016-2018 patients were subjected to external temporal validation. A single-factor COX regression model was first used to screen for prognostic correlates. Then a multi-factor COX regression model was used to screen for independent risk factors. A nomogram was constructed by using independent risk factors. The accuracy and reliability of the nomogram were examined using calibration curves, consistency index (C-index), and area under subjects (AUC) as operational characteristic curves. Decision curve analysis (DCA) was performed to evaluate the clinical value of the prediction model.
Results: A total of 11,557 patients were included in this study, divided into training set (N = 4,712), validation set
Conclusions: We established a nomogram to predict the CSS and OS in elderly MIBC patients undergoing radical cystectomy. After internal cross-validation and external validation, the nomogram prediction model showed good accuracy and reliability, and the DCA results showed that the nomogram has good clinical value. In addition, this study gave good suggestions on whether radiotherapy or chemotherapy is necessary for radical cystectomy in elderly MIBC patients.
Keywords
INTRODUCTION
Bladder cancer (BCa) ranks fourth among men, with 81,180 new cases of bladder cancer expected in the United States in 2022 and 17,100 deaths expected from bladder cancer[1]. The development of bladder cancer is associated with various factors such as age, marital race, gender, and smoking. The degree of tumor infiltration can be divided into muscle-invasive bladder cancer (MIBC) and non-muscle-invasive bladder cancer (NMIBC). MIBC is defined as patients with stage T2 and higher bladder cancer[2,3]. The survival rate of patients with MIBC is low, regardless of treatment[4], with a 5-year survival rate of approximately 45%. Radical cystectomy with neoadjuvant chemotherapy is considered the standard of care for MIBC and can result in an overall 5-year survival (OS) of more than 50% for patients suitable for it[5]. Surgical treatment of MIBC includes radical cystectomy, partial cystectomy, and urinary diversion.
Nevertheless, radical cystectomy remains the gold standard for treating patients with MIBC[6]. Despite radical cystectomy, approximately half of the patients with MIBC develop metastatic disease and die from it within 2 years of diagnosis, with a 60% risk of death within 5 years[7,8]. Patients with NMIBC predominantly have bladder preservation, but 32% of patients with NMIBC[9] have been reported to develop invasive bladder cancer after treatment. Survival of cancer patients is closely related to various factors, including age, gender, race, ethnicity, marriage, tumor stage, and treatment modality. Therefore, finding independent risk factors associated with survival after radical cystectomy in patients with MIBC is essential to improve the survival rate of patients with MIBC.
Related work
The nomogram is a new graphical representation of the statistical prediction model commonly used to predict patient prognostic-related factors[10]. The nomogram is superior to conventional TNM staging and can incorporate a variety of clinicopathological factors other than the TNM stage. The nomogram has been used in various urological tumors, including kidney and Wilblastoma[11,12]. Some researchers have also developed nomograms of BCa, such as Wu et al., who developed radiographic nomograms and validated them for preoperative prediction of BCa lymph node (LN) metastasis[13]. Wang et al. have developed and validated reliable nomograms to estimate overall survival (OS) for BCa, and it shows better discrimination than the 8th edition of the tumor-lymph node-metastasis (TNM) staging system[14].
BCa is a geriatric disease whose onset is closely related to age. The literature shows more than 80% of BCa patients are over 65 years old[15]. Elderly patients are prone to cardiovascular disease and respiratory system disease comorbidities. Therefore their OS may be affected by comorbidities. So the OS of elderly patients cannot respond to specific death caused by cancer. Cancer-specific survival (CSS) can react to certain death caused by the tumor, so predicting the prognostic factors related to CSS in cancer patients is more important to reduce the mortality of cancer patients. The SEER database, the National Cancer Registry, contains data from 18 cancer centers, covers more than 30% of the population, and can respond to cancer big data situations. Currently, it is a trend to develop nomograms based on SEER database data.
To our knowledge, no model can predict the independent risk factors associated with outcomes in older MIBC patients over 65 years undergoing radical cystectomy. Therefore, this study aims to develop and verify using the SEER database. This new nomogram can be used to predict postoperative CSS and OS in elderly patients with MIBC and to assist clinicians and patients in clinically assisted decision-making.
PATIENTS AND METHODS
Dataset description
We extracted information on patients diagnosed with muscle-invasive bladder cancer between 2004-2018 in the SEER database, and all patients were older than 65 years old and underwent radical cystectomy. Data from the SEER database are publicly available, and patient information is hidden, so the SEER database-based research does not require ethical approval and patient-informed unification. This study followed the research guidelines published by the SEER database.
Preprocessing
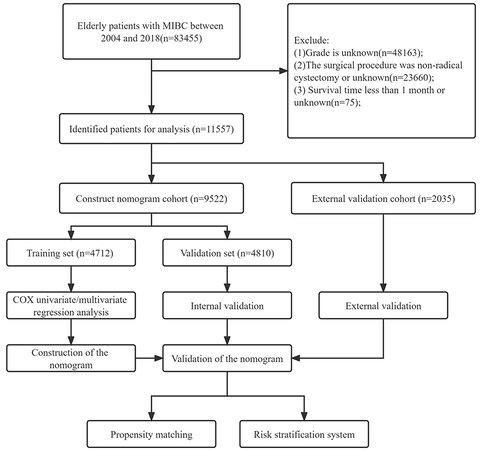
This study included all clinicopathological information for elderly MIBC patients treated in radical resection. It included patient gender, race, age, marital status, TNM stage, Grade grade, radiotherapy, and chemotherapy, and the follow-up results included survival status: survival time and cause of death. Patient inclusion criteria were: (1) a pathological diagnosis of MIBC; (2) an age of over 65 years old; and (3) a radical cystectomy. Patient discharge criteria: (1) age < 65 years; (2) Grade grade unknown; (3) T stage T1 or unknown; (4) N stage unknown; (5) M stage unknown; (6) surgery is not radical resection or unknown; and (7) survival time < 1 month or unknown. Patients’ inclusion and discharge criteria are shown in Figure 1.
Figure 1. Flow chart of inclusion and exclusion of elderly patients with MIBC. Patients in the training set were used to screen independent risk factors and establish a nomogram. The accuracy of the model was checked using a validation set and an external validation set. Finally, a risk stratification system was established to identify high-risk patients and applied clinically by propensity matching.
Nomogram construction and validation
With the 2004-2015 patients used for nomogram development and internal validation, patients were randomized to the training and validation set with a ratio of 1:1. Patients from 2016-2018 were used for external validation in time. Univariate COX regression analysis was used to screen for the influencing factors associated with patient CSS and OS, and multivariate COX regression analysis was used to screen for independent risk factors. Nomograms were then constructed using independent risk factors to predict CSS and OS at 1, 3, and 5 years in elderly MIBC patients undergoing radical cystectomy. The accuracy of this predictive model was tested using 1,000 bootstrap samples. Using the concordance index (C-index) and the area under the subject operating characteristic curve (AUC) to test the recognition ability of the predictive model, the AUC was performed separately for the training set, validation set, and externally validated patients.
Clinical application
The potential clinical value of the nomogram prediction model was tested using the decision analysis curve (DCA). In addition, in calculating the risk value for each patient. The receiver operating characteristic (ROC) curve was used to determine the cut-off value. Patients were divided into high-risk and low-risk groups using cut-off values. The high and low-risk groups’ survival differences were compared using the Kaplan-Meier (K-M) curves and the log-rank test. In addition, we also analyzed the survival differences between chemotherapy and radiotherapy methods in all patients and again analyzed the effects of chemotherapy and radiotherapy on patient survival after a 1:1 propensity match.
Statistical analysis
Continuous variables are described using the mean and standard deviation, e.g., age. Chi-square tests or non-parametric U-tests were used for comparison between groups. Frequently, other categorical variables were described by chi-square tests and were used for intergroup comparisons. The Cox regression model was used to analyze patient outcome-related risk factors, and the log-rank test was used to analyze patient differences in survival. The R software version 4.1.0 and SPSS 26.0 were used for statistical analysis. The R packages used include the “DynNom”, “RMS”, “survival”, “matching”, and “ggDCA”. P values less than 0.05 were considered statistically significant.
RESULTS
Clinical features of the patients
11,557 elderly MIBC patients undergoing radical cystectomy between 2004 and 2018 and 9,522 patients between 2004-2015 were randomly assigned to the training set (N = 4,712) and validation set (N = 4,810) for nomogram development and internal validation, respectively. In time, 2,035 patients in 2016-2018 were used for external validation. The mean age of the patients was 74.3 ± 6.13 years, with 73.7% male, 90.1% white, 64.1% married, T2 stage 42%, T3-T4 stage 58%, M0 94.6%, N0 73.9%, 37.3% receiving chemotherapy, and only 3.7% of patients receiving radiotherapy. No basic information is significant between the two groups; the detailed results are shown in Table 1.
Clinicopathological characteristics of elderly MIBC patients with radical cystectomy
| All | Training cohort | Validation cohort | P | |
| N = 9,522 | N = 4,712 | N = 4,810 | ||
| Age | 74.3 (6.13) | 74.3 (6.12) | 74.3 (6.14) | 0.625 |
| Sex: | 0.731 | |||
| Male | 7,020 (73.7%) | 3,466 (73.6%) | 3,554 (73.9%) | |
| Female | 2,502 (26.3%) | 1,246 (26.4%) | 1,256 (26.1%) | |
| Race: | 0.780 | |||
| White | 8,577 (90.1%) | 4,252 (90.2%) | 4,325 (89.9%) | |
| Black | 482 (5.06%) | 231 (4.90%) | 251 (5.22%) | |
| Other | 463 (4.86%) | 229 (4.86%) | 234 (4.86%) | |
| Marital: | 0.452 | |||
| No/unknown | 3,419 (35.9%) | 1,710 (36.3%) | 1,709 (35.5%) | |
| Married | 6,103 (64.1%) | 3,002 (63.7%) | 3,101 (64.5%) | |
| T: | 0.969 | |||
| T2 | 3,996 (42.0%) | 1,976 (41.9%) | 2,020 (42.0%) | |
| T3-4 | 5,526 (58.0%) | 2,736 (58.1%) | 2,790 (58.0%) | |
| M: | 0.217 | |||
| M0 | 9,005 (94.6%) | 4,442 (94.3%) | 4,563 (94.9%) | |
| M1 | 517 (5.43%) | 270 (5.73%) | 247 (5.14%) | |
| N: | 0.751 | |||
| N0 | 7,037 (73.9%) | 3,475 (73.7%) | 3,562 (74.1%) | |
| N1-3 | 2,485 (26.1%) | 1,237 (26.3%) | 1,248 (25.9%) | |
| Chemotherapy: | 0.589 | |||
| No/unknown | 5,974 (62.7%) | 2,943 (62.5%) | 3,031 (63.0%) | |
| Yes | 3,548 (37.3%) | 1,769 (37.5%) | 1,779 (37.0%) | |
| Radiation: | 0.719 | |||
| No/unknown | 9,168 (96.3%) | 4,533 (96.2%) | 4,635 (96.4%) | |
| Yes | 354 (3.72%) | 179 (3.80%) | 175 (3.64%) | |
| CSS: | 0.553 | |||
| Dead | 4,557 (47.9%) | 2,270 (48.2%) | 2,287 (47.5%) | |
| Alive | 4,965 (52.1%) | 2,442 (51.8%) | 2,523 (52.5%) | |
| Survival months | 46.5 (43.1) | 46.6 (43.4) | 46.5 (42.7) | 0.899 |
| Status: | 0.598 | |||
| Dead | 6,774 (71.1%) | 3,340 (70.9%) | 3,434 (71.4%) | |
| Alive | 2,748 (28.9%) | 1,372 (29.1%) | 1,376 (28.6%) |
Univariate and multivariate COX regression analysis
We first used univariate COX regression analysis to screen risk factors affecting patient CSS and OS in the training set, based on this analysis using multivariate COX regression analysis to screen independent risk factors. The univariate COX regression analysis showed that the risk factors for CSS and OS included age, race, marital status, TNM stage, and chemoradiotherapy. The multivariate COX regression analysis results showed that marriage was not an independent risk factor affecting CSS and OS. Detailed results are presented in Tables 2 and 3.
Univariate and multivariate analyses of CSS in training cohort
| Univariate | Multivariate | |||||
| HR | 95%CI | P | HR | 95%CI | P | |
| Age | 1.03 | 1.02-1.03 | < 0.001 | 1.021 | 1.014-1.028 | < 0.001 |
| Race: | ||||||
| White | ||||||
| Black | 1.31 | 1.09-1.57 | 0.004 | 1.252 | 1.043-1.502 | 0.016 |
| Other | 0.82 | 0.67-1.01 | 0.06 | 0.802 | 0.653-0.985 | 0.036 |
| Marital: | ||||||
| No/unknown | ||||||
| Married | 0.88 | 0.8-0.95 | 0.002 | |||
| T: | ||||||
| T2 | ||||||
| T3-4 | 2.76 | 2.51-3.02 | < 0.001 | 2.232 | 2.026-2.459 | < 0.001 |
| M: | ||||||
| M0 | ||||||
| M1 | 3.05 | 2.64-3.52 | < 0.001 | 2.12 | 1.827-2.46 | < 0.001 |
| N: | ||||||
| N0 | ||||||
| N1-3 | 2.43 | 2.23-2.65 | < 0.001 | 1.899 | 1.731-2.083 | < 0.001 |
| Chemotherapy: | ||||||
| No/unknown | ||||||
| Yes | 0.84 | 0.77-0.91 | < 0.001 | 0.711 | 0.649-0.78 | < 0.001 |
| Radiation: | ||||||
| No/unknown | ||||||
| Yes | 1.73 | 1.44-2.09 | < 0.001 | 1.38 | 1.142-1.668 | 0.001 |
Univariate and multivariate analyses of OS in training cohort
| Univariate | Multivariate | |||||
| HR | 95%CI | P | HR | 95%CI | P | |
| Age | 1.04 | 1.03-1.04 | < 0.001 | 1.031 | 1.025-1.037 | < 0.001 |
| Race: | ||||||
| White | ||||||
| Black | 1.32 | 1.13-1.53 | < 0.001 | 1.242 | 1.067-1.447 | 0.005 |
| Other | 0.81 | 0.69-0.96 | 0.017 | 0.806 | 0.681-0.954 | 0.012 |
| Marital: | ||||||
| No/unknown | ||||||
| Married | 0.84 | 0.78-0.9 | < 0.001 | |||
| T: | ||||||
| T2 | ||||||
| T3-4 | 2.24 | 2.08-2.4 | < 0.001 | 1.895 | 1.756-2.045 | < 0.001 |
| M: | ||||||
| M0 | ||||||
| M1 | 2.52 | 2.21-2.87 | < 0.001 | 1.903 | 1.661-2.18 | < 0.001 |
| N: | ||||||
| N0 | ||||||
| N1-3 | 1.98 | 1.84-2.13 | < 0.001 | 1.647 | 1.521-1.784 | < 0.001 |
| Chemotherapy: | ||||||
| No/unknown | ||||||
| Yes | 0.78 | 0.72-0.84 | < 0.001 | 0.699 | 0.648-0.754 | < 0.001 |
| Radiation: | ||||||
| No/unknown | ||||||
| Yes | 1.61 | 1.37-1.89 | < 0.001 | 1.419 | 1.203-1.673 | < 0.001 |
Development and validation of the nomograms
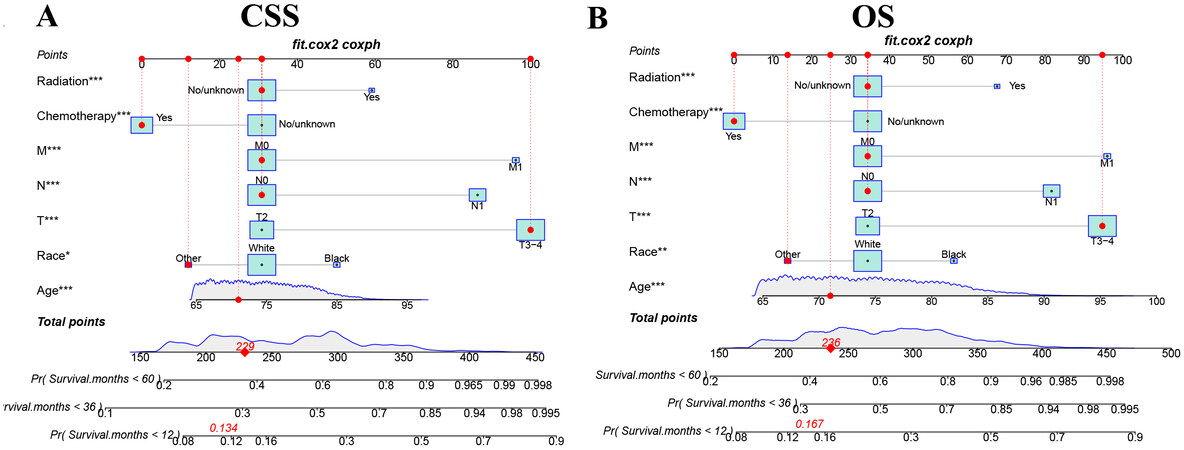
We used independent risk factors to construct a nomogram that could predict the CSS and OS in elderly MIBC patients undergoing radical cystectomy [Figure 2]. The nomograms of CSS and OS show older age and higher TNM stage and lower CSS and OS; blacks have the lowest CSS and OS, followed by white people; chemotherapy is a protective factor for CSS and OS, and radiotherapy is a risk factor.
Figure 2. The nomograms for predicting 1, 3, and 5-year CSS and OS in elderly MIBC patients with radical cystectomy. (A) The nomogram for predicting CSS. (B) The nomogram for predicting OS. *P < 0.05; **P < 0.01; ***P < 0.001.
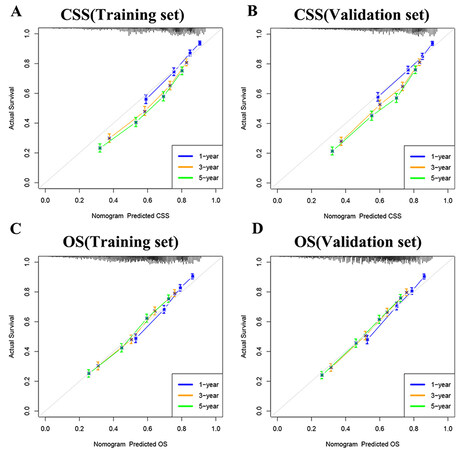
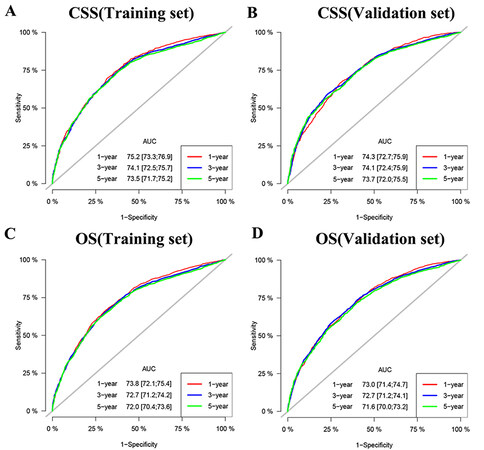
Furthermore, we tested the model through internal and external validation in time. The C index of the CSS training set and validation set is 0.692 (95%CI: 0.680-0.704) and 0.690 (95%CI: 0.678-0.702, respectively), and the C index of the OS training set and validation set is 0.674 (95%CI: 0.664-0.684) and
Figure 3. Calibration curve of the nomograms for predicting 1-,3-,5-year CSS and OS in elderly MIBC patients with radical cystectomy. (A) Calibration curve of the nomograms for predicting CSS in the training set. (B) Calibration curve of the nomograms for predicting CSS in the validation set. (C) Calibration curve of the nomograms for predicting OS in the training set. (D) Calibration curve of the nomograms for predicting OS in the validation set. The horizontal axis is the predicted value in the nomogram, and the vertical axis is the observed value.
Clinical application of the nomograms
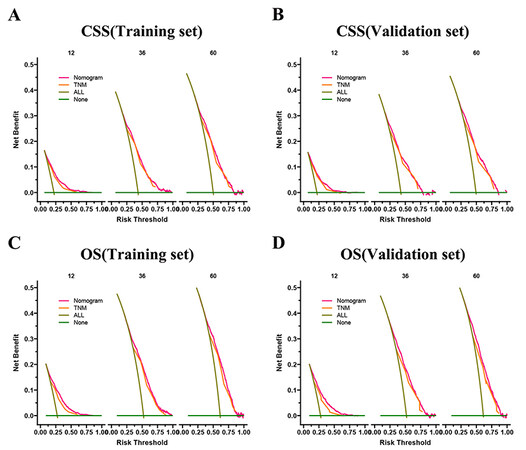
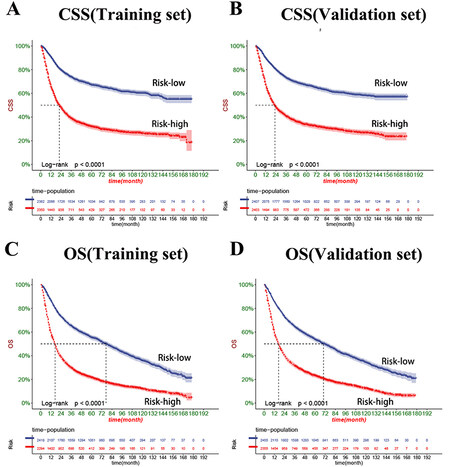
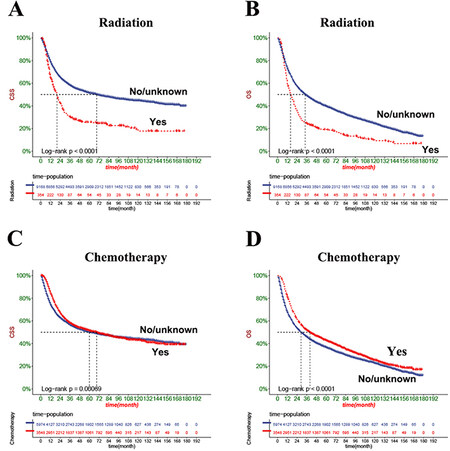
The DCA results showed that both OS and CSS nomograms had good clinical value [Figure 5]. Moreover, our established nomogram prediction models of CSS and OS are better predicted than the conventional TNM staging system. Furthermore, patient cut-offs calculated from the ROC curve were classified into high-risk and low-risk groups, with a cut-off value of 165.96239 for CSS and 112.675636 for OS. The K-M curve showed that the low-risk group had a high CSS and OS in both the training and validation sets [Figure 6]. The 1-, 3- and 5-year survival rates were 90.1%, 73.4%, and 66.8%, respectively, in the low-risk group of CSS, and the 1-, 3- and 5- years survival rates in a high-risk group for CSS were 66.0%, 39.6%, and 32.7%, respectively. In the low-risk group of OS, the 1-, 3- and 5-year survival rates were 85.8%, 64.7%, and 54.4%, respectively. And 58.6%, 30.4%, and 22.3% in the high-risk group of OS, respectively. In addition, the KM curve showed lower OS and CSS in patients receiving radiotherapy, suggesting that radiotherapy is a risk factor for elderly MIBC patients undergoing radical surgery. Second, patients receiving chemotherapy had higher early CSS than patients without chemotherapy.
Figure 5. DCA of the nomograms for predicting CSS and OS. (A) The nomogram for CSS in the training set. (B) The nomogram for CSS in the validation set. (C) The nomogram for OS in the training and training set. (D) The nomogram for OS in the training and validation set.
Figure 6. Kaplan-Meier curves of patients in the low-risk and high-risk groups. (A) The outcome is the KM curve of the high-risk and low-risk groups of CSS in the training set. (B) The outcome is the KM curve of the high-risk and low-risk groups of CSS in the validation set. (C) The outcome is the KM curve of the high-risk and low-risk groups of OS in the training set. (D) The outcome is the KM curve of the high and low-risk groups of OS in the validation set.
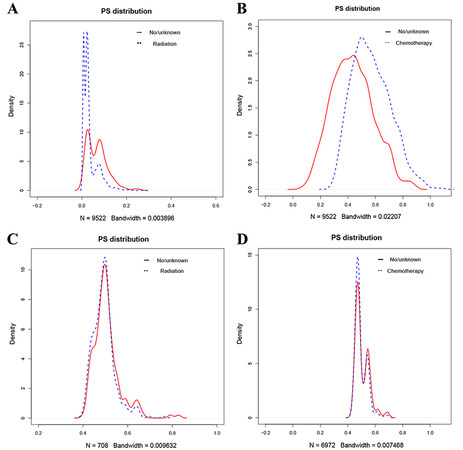
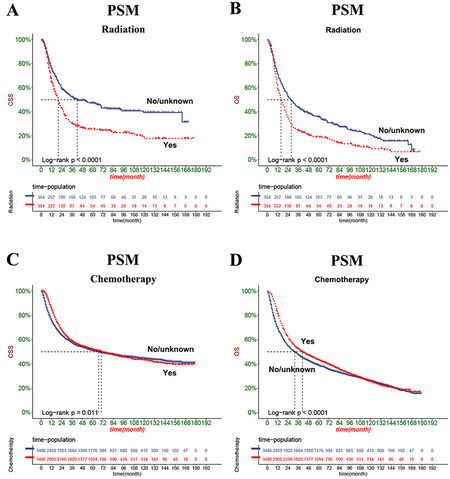
In comparison, advanced CSS was lower than patients without chemotherapy, while patients receiving chemotherapy had higher OS than patients without chemotherapy [Figure 7]. As the survival differences in chemoradiotherapy are incompatible with clinical experience, the consideration may be biased due to other factors. We further excluded the effects of other factors by a 1:1 propensity matching. Results showed differences in patient information except for chemoradiotherapy before and after tendency matching [Figure 8]. After propensity matching, we further analyzed the chemoradiotherapy survival differences by KM curve. The results showed no significant change compared to before propensity matching, considering that the survival difference was due to chemoradiotherapy [Figure 9].
Figure 7. Kaplan-Meier curves of patients with radiotherapy and chemoradiotherapy before the propensity matching. (A) The CSS rate of patients with radiotherapy. (B) The OS rate of patients with radiotherapy. (C) The CSS rate of patients with chemoradiotherapy. (D) The OS rate of patients with chemoradiotherapy.
Figure 8. Density plots of propensity score excluding radiotherapy and chemotherapy before and after matching. (A) Density plots of propensity score excluding radiotherapy before matching. (B) Density plots of propensity score excluding chemotherapy before matching. (C) Density plots of propensity score excluding radiotherapy after matching. (D) Density plots of propensity score excluding chemotherapy after matching.
Figure 9. Kaplan-Meier curves of patients with radiotherapy and chemoradiotherapy after the propensity matching. (A) The CSS rate of patients with radiotherapy. (B) The OS rate of patients with radiotherapy. (C) The CSS rate of patients with chemoradiotherapy. (D) The OS rate of patients with chemoradiotherapy.
DISCUSSION
The degree of muscle infiltration distinguishes MIBC, including stage ≥ T2, which accounts for approximately 1/4 of newly diagnosed BCa[2]. Old age is a high incidence of cancer, and most deaths from BCa occur in old age; cystectomy is a complex surgical procedure with a high risk of complications. A
Currently, radiation therapy is one of the main treatment modalities. A recent Swedish study reported that more than half (57%) of MIBC patients received radiation therapy despite the slow incidence of MIBC[18]. For patients with MIBC who are not eligible for surgery, “radical” radiotherapy is recommended as an alternative to radical cystectomy[19,20]. Radiotherapy alone or combined with surgery and chemotherapy is used to treat a wide range of cancers in more than 50% of patients with cancer[21]. 77% of lung cancer patients have evidence-based indications for radiotherapy[22]. In addition, two large trials support the role of radiotherapy for patients with low-risk breast cancer[23]. A randomized trial showed that adjuvant sequential radiotherapy plus chemotherapy improved local recurrence-free survival compared with adjuvant chemotherapy alone in patients with locally advanced bladder cancer[24]. Radiotherapy has been reported as an adjuvant treatment for patients with MIBC combined with radical cystectomy. It can reduce local and local recurrence after cystectomy while improving survival[25]. However, it is controversial whether MIBC in the elderly must be combined with radiation therapy after surgery.
Single-center studies often lead to bias due to small sample sizes. However, a nomogram constructed based on large data can avoid bias due to small data size. Our column line graphs constructed based on the SEER database showed that radiotherapy was an independent risk factor for developing CSS and OS in elderly MIBC patients who underwent radical resection. The KM curves also showed that CSS and OS were lower in radiotherapy patients. To exclude bias in the results for factors other than radiotherapy, we also performed a 1:1 propensity matching of patients. We found that radiotherapy still favored the prognosis of elderly MIBC patients undergoing radical resection. Considering the side effects of radiotherapy, many patients who underwent radiotherapy had impaired renal function or performed poorly[26]. Therefore, we do not recommend radiotherapy for elderly MIBC patients undergoing radical cystectomy.
Chemotherapy is also an effective treatment modality for solid malignant tumors. In recent years, neoadjuvant chemotherapy has been administered to patients with MIBC undergoing cystectomy in many academic centers[27]. The literature reports that chemotherapy improves OS and reduces bladder cancer-specific mortality in patients with bladder cancer[28]. A meta-analysis of neoadjuvant chemotherapy for muscle-invasive bladder cancer suggested that adjuvant chemotherapy should be the standard of care for MIBC[29]. A multicenter phase 3 clinical trial showed that chemotherapy combined with radiotherapy significantly improved the prognosis of patients with MIBC and reduced recurrence compared with radiotherapy alone[26]. However, several prospective and observational studies have shown that chemotherapy cure rates are higher in patients in complete pathological remission (ypT0N0), but survival rates are poorer in patients with stage T2 or higher[30,31]. Therefore, it remains controversial whether to perform postoperative chemotherapy in MIBC patients. We constructed a columnar plot showing that chemotherapy was a protective factor for OS and CSS. However, KM curve analysis showed that chemotherapy favored short-term CSS and discouraged long-term CSS in elderly MIBC patients undergoing radical resection. We performed further propensity matching using KM curves again to analyze chemotherapy survival differences, and the results were the same as before propensity matching. Therefore, for elderly MIBC patients undergoing radical cystectomy, we suggest that chemotherapy can be administered if necessary, but their long-term complications and outcomes should be closely monitored.
The TNM staging system is a relatively classical method for determining tumor prognosis. The invasive muscle status of BCa patients is critical for treatment decisions in patients with[6]. The nomogram OS prediction map for bladder cancer patients developed by Wang et al. showed that patient OS correlated with the TNM stage and higher TNM stage and lower OS[14]. The bladder migratory cell carcinoma constructed by Huang et al. found that the higher the T and N stages, the lower the CSS[32]. Our column line graph showed that the TNM stage was an independent risk factor for developing CSS and OS in elderly patients undergoing radical cystectomy. The higher the TNM stage, the lower the CSS and OS of patients, consistent with previous results.
Older adults are a high-risk age group for BCa, and age is also a prognostic factor for BCa. Lenis et al. showed that advanced age leads to bladder cancer progression[33]. In addition, studies have confirmed that bladder cancer metastases increase with the patient’s age[34]. Our line graph shows that age is an independent risk factor for developing CSS and OS in elderly MIBC patients undergoing radical resection, and the older these patients are, the lower the CSS and OS.
Race is strongly associated with the incidence of many cancers. Studies have shown that although the incidence of MIBC is lower in Blacks than Whites, the 5-year overall survival rate is also lower in Blacks than in Whites[35]. Our line graph shows that race is strongly associated with CSS and OS in older MIBC patients who underwent radical resection. Black patients had the worst prognosis for CSS and OS, followed by whites, with other races having a better prognosis. Recent studies have shown that black patients with muscle-invasive bladder cancer (MIBC) are 21% less likely to receive guideline therapy (GBT), which may explain the worst survival rates in black patients[36].
In conclusion, based on the SEER database, this study constructed a line graph of CSS and OS in elderly MIBC patients after radical cystectomy. Our study incorporated most of the critical variables related to prognosis and promptly confirmed the nomogram’s accuracy by internal and external validation. Also, DCA showed that the predictive model we constructed was superior to the conventional TNM staging system.
The most important finding of this study was that radiotherapy was detrimental to both CSS and OS in elderly MIBC patients undergoing radical cystectomy. Also, chemotherapy was a protective factor for CSS in the short term and a detrimental factor in the long term. We reached the same conclusion after propensity matching. These findings update our previous views and may provide a fundamental theoretical basis for whether to administer radiotherapy and chemotherapy to patients undergoing radical cystectomy in the future.
However, there are some limitations to our study. First, although the study based on the SEER database included many variables related to prognosis, some key factors, such as smoking and obesity, were still missing to include all variables related to prognosis. Second, our study was retrospective and could not avoid selection bias. Finally, in the inclusion and exclusion criteria, we excluded patients with incomplete clinicopathological data and follow-up data, which may also lead to some bias in the representativeness of the included sample.
In future studies, we will first include patient information from the updated SEER database (after 2019) and perform statistical analysis after the same inclusion criteria for further internal validation. Meanwhile, we will collect elderly MIBC surgery patients treated at multiple study centers in Yunnan Province and perform an external validation of this prediction model based on the same inclusion and exclusion criteria to further demonstrate the validity of our constructed nomogram.
In summary, this study is the first to examine the factors influencing the occurrence of CSS and OS after radical cystectomy in elderly MIBC patients. We found that radiotherapy, chemotherapy, T-stage, N-stage, M-stage, race, and age were independent risk factors affecting patients’ CSS and OS. Based on these independent risk factors, we constructed a nomogram prediction model that could predict CSS and OS after radical cystectomy in elderly MIBC patients. The DCA results were better than the traditional TNM staging system. They had good clinical value, providing better suggestions for clinicians to diagnose and treat elderly MIBC patients undergoing radical cystectomy, whether they need radiotherapy or chemotherapy after surgery.
DECLARATION
Authors’ contributionsDesigned the study: Zhanghuang C
Collected and analyzed the data: Zhanghuang C, Zhang Z, Wang J, Xie Y, Ji F, Tang H
Drafted the initial manuscript: Zhanghuang C
Revised the article critically: Zhanghuang C, Jiang H, Yang Z, Yan B
Reviewed and edited the article: Zhanghuang C, Zhang Z, Yao Z, Wu C
All authors approved the final manuscript.
Availability of data and materialsThe SEER data analyzed in this study is available at https://seer.Cancer.gov/.
Financial support and sponsorshipThe study was supported by: Scientific Research Foundation of Education Department of Yunnan Province (No. 2023J0295), Kunming Medical University Joint Project of Department of Science and Technology of Yunnan Province (No. 02301AY070001-108); Kunming City Health Science and Technology Talent “1000” training Project (No. 2020-SW (Reserve)-112), Kunming Health and Health Commission Health Research Project (No. 2020-0201-001), Kunming Medical Joint Project of Yunnan Science and Technology Department (No. 202001 AY070001-271), and Open Research Fund of Clinical Research Center for Children’s Health and Diseases of Yunnan Province (2022-ETYY-YJ-03). The funding bodies played no role in the study’s design and collection, analysis and interpretation of data, and writing the manuscript.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateThe data of this study is obtained from the SEER database. The patients’ data is public and anonymous, so this study does not require ethical approval and informed consent.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
Supplementary MaterialsREFERENCES
2. Cumberbatch MGK, Jubber I, Black PC, et al. Epidemiology of bladder cancer: a systematic review and contemporary update of risk factors in 2018. Eur Urol 2018;74:784-95.
3. Burger M, Oosterlinck W, Konety B, et al. ICUD-EAU international consultation on bladder cancer 2012: non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol 2013;63:36-44.
4. Rödel C, Grabenbauer GG, Kühn R, et al. Combined-modality treatment and selective organ preservation in invasive bladder cancer: long-term results. J Clin Oncol 2002;20:3061-71.
5. Arcangeli G, Strigari L, Arcangeli S. Radical cystectomy versus organ-sparing trimodality treatment in muscle-invasive bladder cancer: a systematic review of clinical trials. Crit Rev Oncol Hematol 2015;95:387-96.
6. Witjes JA, Bruins HM, Cathomas R, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol 2021;79:82-104.
7. Witjes JA, Compérat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol 2014;65:778-92.
8. Shariat SF, Karakiewicz PI, Palapattu GS, et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the Bladder Cancer Research Consortium. J Urol 2006;176:2414-22; discussion 2422.
9. Cumberbatch MGK, Foerster B, Catto JWF, et al. Repeat transurethral resection in non-muscle-invasive bladder cancer: a systematic review. Eur Urol 2018;73:925-33.
10. Thandapani S, Mahaboob MI, Iwendi C, et al. IoMT with deep CNN: AI-based intelligent support system for pandemic diseases. Electronics 2023;12:424.
11. Chou CY, Shu KH, Chen HC, et al. Development and validation of a nomogram for urothelial cancer in patients with chronic kidney disease. Sci Rep 2019;9:3473.
12. Pan Z, You H, Bu Q, et al. Development and validation of a nomogram for predicting cancer-specific survival in patients with Wilms’ tumor. J Cancer 2019;10:5299-305.
13. Wu S, Zheng J, Li Y, et al. A radiomics nomogram for the preoperative prediction of lymph node metastasis in bladder cancer. Clin Cancer Res 2017;23:6904-11.
14. Wang J, Wu Y, He W, Yang B, Gou X. Nomogram for predicting overall survival of patients with bladder cancer: a population-based study. Int J Biol Markers 2020;35:29-39.
15. Tao L, Pan X, Zhang L, et al. Marital status and prognostic nomogram for bladder cancer with distant metastasis: a SEER-based study. Front Oncol 2020;10:586458.
16. Novara G, De Marco V, Aragona M, et al. Complications and mortality after radical cystectomy for bladder transitional cell cancer. J Urol 2009;182:914-21.
17. Schiffmann J, Gandaglia G, Larcher A, et al. Contemporary 90-day mortality rates after radical cystectomy in the elderly. Eur J Surg Oncol 2014;40:1738-45.
18. Westergren DO, Gårdmark T, Lindhagen L, Chau A, Malmström PU. A nationwide, population based analysis of patients with organ confined, muscle invasive bladder cancer not receiving curative intent therapy in Sweden from 1997 to 2014. J Urol 2019;202:905-12.
19. Gospodarowicz MK, Rider WD, Keen CW, et al. Bladder cancer: long-term follow-up results of patients treated with radical radiation. Clin Oncol 1991;3:155-61.
20. Fosså SD, Wæhre H, Aass N, Jacobsen A, Olsen DR, Ous S. Bladder cancer definitive radiation therapy of muscle-invasive bladder cancer: a retrospective analysis of 317 patients. Cancer 1993;72:3036-43.
21. Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005;104:1129-37.
22. Vinod SK, Hau E. Radiotherapy treatment for lung cancer: current status and future directions. Respirology 2020;25 Suppl 2:61-71.
23. Haussmann J, Corradini S, Nestle-Kraemling C, et al. Recent advances in radiotherapy of breast cancer. Radiat Oncol 2020;15:71.
24. Zaghloul MS, Christodouleas JP, Smith A, et al. Adjuvant sandwich chemotherapy plus radiotherapy vs adjuvant chemotherapy alone for locally advanced bladder cancer after radical cystectomy: a randomized phase 2 trial. JAMA Surg 2018;153:e174591.
25. Pignot G, Sargos P. Adjuvant radiotherapy after radical cystectomy for muscle-invasive bladder cancer. Prog Urol 2021;31:158-68.
26. James ND, Hussain SA, Hall E, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med 2012;366:1477-88.
27. Reardon ZD, Patel SG, Zaid HB, et al. Trends in the use of perioperative chemotherapy for localized and locally advanced muscle-invasive bladder cancer: a sign of changing tides. Eur Urol 2015;67:165-70.
28. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859-66.
29. Yin M, Joshi M, Meijer RP, et al. Neoadjuvant chemotherapy for muscle-invasive bladder cancer: a systematic review and two-step meta-analysis. Oncologist 2016;21:708-15.
30. Zargar H, Espiritu PN, Fairey AS, et al. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol 2015;67:241-9.
31. Zargar H, Zargar-Shoshtari K, Lotan Y, et al. Final pathological stage after neoadjuvant chemotherapy and radical cystectomy for bladder cancer-does pT0 predict Better survival than pTa/Tis/T1? J Urol 2016;195:886-93.
32. Huang C, Zhou W, Song P, Yuan N. Comparison of different prognostic models for predicting cancer-specific survival in bladder transitional cell carcinoma. Future Oncol 2019;15:851-64.
34. Rosiello G, Palumbo C, Deuker M, et al. Sex- and age-related differences in the distribution of bladder cancer metastases. Jpn J Clin Oncol 2021;51:976-83.
35. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin 2019;69:211-33.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Zhanghuang C, Zhang Z, Jiang H, Wang J, Yao Z, Ji F, Wu C, Yang Z, Xie Y, Tang H, Yan B. Development and validation of a survival nomogram for muscle-invasive bladder cancer in the elderly: using competing risk models and propensity matching to apply the prediction tool. J Cancer Metastasis Treat 2023;9:18. http://dx.doi.org/10.20517/2394-4722.2023.20
AMA Style
Zhanghuang C, Zhang Z, Jiang H, Wang J, Yao Z, Ji F, Wu C, Yang Z, Xie Y, Tang H, Yan B. Development and validation of a survival nomogram for muscle-invasive bladder cancer in the elderly: using competing risk models and propensity matching to apply the prediction tool. Journal of Cancer Metastasis and Treatment. 2023; 9: 18. http://dx.doi.org/10.20517/2394-4722.2023.20
Chicago/Turabian Style
Zhanghuang, Chenghao, Zhaoxia Zhang, Hongchao Jiang, Jinkui Wang, Zhigang Yao, Fengming Ji, Chengchuang Wu, Zhen Yang, Yucheng Xie, Haoyu Tang, Bing Yan. 2023. "Development and validation of a survival nomogram for muscle-invasive bladder cancer in the elderly: using competing risk models and propensity matching to apply the prediction tool" Journal of Cancer Metastasis and Treatment. 9: 18. http://dx.doi.org/10.20517/2394-4722.2023.20
ACS Style
Zhanghuang, C.; Zhang Z.; Jiang H.; Wang J.; Yao Z.; Ji F.; Wu C.; Yang Z.; Xie Y.; Tang H.; Yan B. Development and validation of a survival nomogram for muscle-invasive bladder cancer in the elderly: using competing risk models and propensity matching to apply the prediction tool. J. Cancer. Metastasis. Treat. 2023, 9, 18. http://dx.doi.org/10.20517/2394-4722.2023.20
About This Article
Copyright
Data & Comments
Data

 Cite This Article 14 clicks
Cite This Article 14 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.