The past, present, and future of targeted therapeutic approaches in patients with diffuse pleural mesotheliomas
Abstract
Despite our growing understanding of the genomic landscape of diffuse pleural mesotheliomas (DPM), there has been limited success in targeted therapeutic strategies for the disease. This review summarizes attempts to develop targeted therapies in DPM, focusing on the following targets being clinically explored in recent and ongoing clinical trials: vascular endothelial growth factor, mesothelin, BRCA1-associated protein 1, Wilms tumor 1 protein, NF2/YAP/TAZ, CDKN2, methylthioadenosine phosphorylase, v-domain Ig suppressor T-cell activation, and argininosuccinate synthetase 1. Although preclinical data for these targets are promising, few have efficaciously translated to benefit our patients. Future efforts should seek to expand the availability of preclinical models that faithfully recapitulate DPM biology, develop clinically relevant biomarkers, and refine patient selection criteria for clinical trials.
Keywords
INTRODUCTION
Diffuse pleural mesothelioma (DPM) is an aggressive malignancy of the mesothelial lining of the pleural cavity with unacceptably poor outcomes, with less than 10% of patients surviving past 5 years[1]. Approximately 3,300 patients are diagnosed annually with DPM in the United States, and globally, the incidence continues to rise in association with asbestos exposure[2-5]. Despite recent advances, DPM remains a recalcitrant disease, with even patients with early-stage disease having a high rate of recurrence despite aggressive multimodality therapy[6,7].
DPM has been the subject of extensive genomic analyses making it a rich field for the pursuit of targeted therapy. Whole exome sequencing of malignant mesotheliomas identifies significant mutational burdens in BAP1, NF2, TP53, and SETD2, amongst many others[8]. In addition to this extensive genomic profiling, immunohistochemistry (IHC) has confirmed protein targets for investigation, such as WT1[9,10], mesothelin[11,12], BAP1[13-15], and VISTA[16,17] and histologic subtype has shown both prognostic and possibly predictive implications[18]. As we gain further insights into the molecular landscape of mesothelioma, there is hope that targeted therapeutic strategies akin to those seen in non-small cell lung cancers over the past two decades will soon follow[19].
However, despite the myriad of efforts to drug these promising targets, in 2022, there exists not a single FDA-approved targeted therapy for patients with DPM. Unresectable/metastatic DPM is traditionally treated with systemic therapies. There are currently only two approved options for patients with DPM, both of which are in the first-line (1L) setting: platinum doublet chemotherapy[20] and combination immune checkpoint inhibitor (ICI) therapy with ipilimumab and nivolumab[18]. Both regimens are biomarker agnostic, with the latter showing preferential benefit in non-epithelioid histology (biphasic and sarcomatoid) but remaining an option for all histologic subtypes. There are currently no approved treatment options for patients after progression on 1L therapy.
With our growing understanding of the genomic landscape of mesotheliomas[8,16,21,22], the field is focused on integrating these findings into the care of our patients. Efforts to comprehensively integrate next-generation sequencing (NGS) findings as a prognosticator for patients with mesothelioma are ongoing, typified by the recently published Oncocast-MPM, an open-source, web-accessible, machine-learning risk-prediction model incorporating genomic profiling from patients with DPM which was validated to provide more comprehensive prognostication[22]. At present, there are no approved nor recommended, genomically defined targeted therapies for patients with DPM. Targeted strategies are, at times, used off-label in the proper clinical settings, including in those rare mesotheliomas harboring ALK rearrangements[23-25], NTRK fusions[26], and BRAF V600E[27] mutations. These approaches have not been systematically evaluated as therapeutic drug targets in mesothelioma, and the use of targeted medications for these indications is extrapolated from data in other malignancies[28]. Further exploration of predictive markers and their actionability is needed.
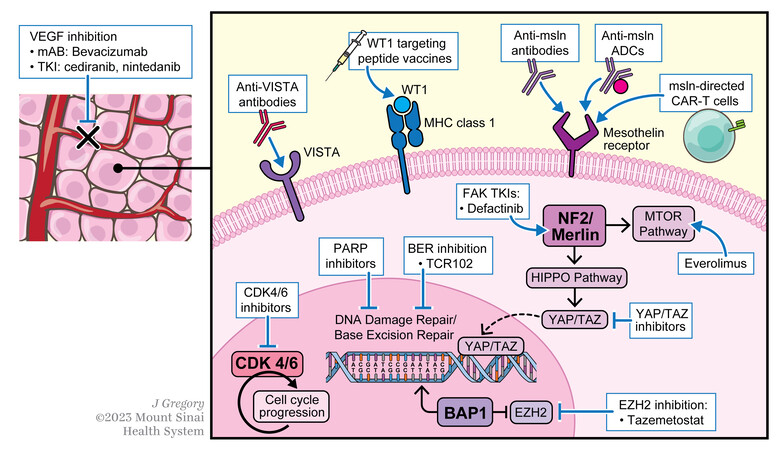
In this review, we will focus on oncogenic targets under active clinical investigation for patients with DPM [Figure 1].
THERAPEUTIC TARGETS OF INTEREST
Vascular endothelial growth factor (VEGF): activation of VEGF and its signaling cascade is critical for tumorigenesis and cell survival[29] across solid tumor types. Inhibition of VEGF signaling has been extensively studied in DPM and is accomplished using targeted antibodies or VEGF tyrosine kinase inhibitors (TKIs). Evidence supporting the use of VEGF inhibitors in DPM has led to their incorporation into National Comprehensive Cancer Network (NCCN) guideline recommendations[30].
VEGF inhibitory (VEGFi) antibodies. The phase 3 MAPS trial randomized 448 patients with DPM to receive cisplatin and pemetrexed with or without the anti-VEGF-A antibody bevacizumab[31]. In this study, median overall survival (OS) was significantly longer in the bevacizumab combination arm (18.8 vs. 16.1 months, HR 0.77, [95%CI: 0.62-0.95], P = 0.02), leading to the inclusion of the triplet therapy regimen in guidelines for 1L recommended treatments in advanced DPM[30,32]. The MAPS trial predates the integration of 1L ipilimumab and nivolumab as a treatment option for patients with DPM and bevacizumab was not allowed in the comparison arm of the CheckMate 743 trial[18]. As such, a direct comparison of platinum/pemetrexed/bevacizumab to dual checkpoint blockade cannot be made. With the integration of immunotherapy into routine practice and the availability of data suggesting an immunomodulatory benefit of VEGFi antibodies with ICI[33], the role of VEGFi antibodies in the 1L setting needs to be re-explored. To that end, the MAPS regimen is currently under investigation in combination with ICI in the 1L phase 3 BEAT-meso trial (NCT03762018) evaluating, in a 1:1 randomization, platinum, pemetrexed, bevacizumab with or without atezolizumab.
Given evolving 1L treatment options in DPM, there is a need to evaluate the utility of VEGFi antibodies in later lines of treatment. The recent double-blind, multicenter, randomized phase 2 RAMES trial examined the inclusion of the anti-VEGFR-2 antibody, ramucirumab, with a standard later-line chemotherapy option, gemcitabine. The study randomized 161 patients to receive gemcitabine with either placebo or ramucirumab. Median OS was significantly longer in the gemcitabine + ramucirumab arm (13.8 vs. 7.5 months, HR 0.71 [95%CI: 0.59-0.85], P = 0.03)[34]. A phase 2 trial of atezolizumab and bevacizumab in the later-line setting for patients with peritoneal mesothelioma, a clinically[35] and genomically[21,36] distinct malignancy of the abdominal cavity, found a promising response rate of 40% with a median duration of response of 12.8 months[37]. These trials lend credence to the argument that VEGF inhibitors are effective treatment options for patients with mesotheliomas. With the movement of ICI into the 1L setting for some patients, it is difficult to know where to incorporate these agents, and in what combinations, without further prospective studies.
VEGF TKIs. In contrast to VEGFi antibodies, VEGF TKIs have failed to demonstrate improved efficacy compared to standard-of-care regimens [Table 1]. The 1L phase 2 SWOG S0905 trial of cediranib (TKI with activity against VEGF and PDGFR) with cisplatin and pemetrexed did not show significant clinical efficacy over chemotherapy alone[38,39] and the phase 2 trial of cediranib monotherapy in patients with ≤ 1 prior line of treatment showed modest clinical benefit but at an intolerable dose level[40]. The 1L phase 3 LUME-Meso trial of cisplatin/pemetrexed with nintedanib (a multi-kinase TKI with activity against PDFG, FGF2, TGFβ, and VEGF) failed to show significant clinical benefit compared to chemotherapy[41], and the phase 2 trial of nintedanib in previously treated patients with DPM did not meet its prespecified progression-free survival (PFS) endpoint[42]. To date, there are no approved nor recommended VEGF TKIs for patients with DPM.
VEGF TKIs in mesothelioma
| NCT ID | Phase | Product | Target patient population | Outcomes | Reference |
| NCT01064648 | 2 | Cisplatin/pemetrexed +/- cediranib | 92 patients with treatment naïve unresectable DPM | Combination vs. chemotherapy alone: PFS: 6.9 vs. 5.6 months (HR 0.77, 95%CI: 0.59-1.02); OS: 10.0 vs. 8.5 months (HR 0.88, 95%CI: 0.65-1.17) | [38,39] |
| NCT00309946 | 2 | Cediranib | 51 patients with DPM and ≤ 1 prior line of therapy | PFS: 1.8 months, OS: 4.4 months. 45 mg dose level improved response rate but intolerable toxicity | [40] |
| NCT01907100 | 3 | Cisplatin/pemetrexed +/- nintedanib | 458 treatment naïve patients with DPM randomized 1:1 to chemotherapy alone and combination | Combination vs. chemotherapy alone: PFS: 6.8 vs. 7.0 months (HR 1.01, 95%CI: 0.79-1.30) | [41] |
| NCT02568449 | 2 | Nintedanib | 20 patients with DPM who previously received chemotherapy | PFS: 1.8 months; OS: 4.2 months | [42] |
Mesothelin: Mesothelin (MSLN) is a membrane-anchored cell surface glycoprotein that is highly expressed in several solid tumors including DPM[11,43]. In preclinical models, MSLN overexpression promotes tumorigenesis and tumor invasion[12,44]. Over the last several decades, there have been several attempts to therapeutically exploit the overexpression of MSLN in DPM using multiple novel constructs, including antibody-drug conjugates (ADCs), immunotoxins, and adoptive cellular therapies[45].
Mesothelin Antibodies. Trials of MSLN targeting antibodies have produced mixed results. The chimeric, humanized IgG1 monoclonal antibody amatuximab was evaluated in a phase 2, single-arm trial in DPM, where it was combined with cisplatin and pemetrexed for six cycles, followed by maintenance amatuximab until disease progression. While the combination was found to be tolerable, the primary endpoint of PFS was not improved in the treatment arm compared to chemotherapy alone, and the construct has not progressed to a phase 3 investigation[46].
SS1P is a mesothelin-targeting antibody attached to a truncated fragment of Pseudomonas exotoxin A. With preclinical data suggesting pseudomonas exotoxin A induces immunogenic cell death in DPM[47], there was a rationale to test whether SS1P could lead to a tolerable therapeutic index and efficacy signal in DPM. The drug was evaluated in a phase 1 trial in combination with cisplatin and pemetrexed for 1L treatment of DPM[48]. In 20 evaluable patients, there was an initial efficacy signal with an overall response rate of 60%; however, neutralizing antidrug antibody formation within the first cycle limited its initial clinical development in DPM. Lymphodepleting regimens (pentostatin/cyclophosphamide) to delay antidrug antibody formation showed some early promise[49] but, at present, are not being actively investigated in larger prospective trials for DPM.
A second-generation immunotoxin, LMB-100, was subsequently designed to be less immunogenic than SS1P[50] to avoid the development of neutralizing antibodies, which were thought to limit single-agent activity. A phase I trial of LMB-100 in advanced MSLN-expressing cancers found that the drug was indeed less immunogenic. However, most patients developed antidrug antibodies after two cycles of the drug, prompting the researchers to conclude that this formulation would have similarly limited single-agent activity as SS1P[51]. While a planned phase II study examining LMB-100 in combination with pembrolizumab (NCT03644550) in the later-line setting was withdrawn due to the evolving 1L landscape after the integration of CheckMate743, a study evaluating the role of normothermic intrapleural LMB-100 after cytoreductive surgery (NCT0537825) is soon to open.
Under the current investigation is the novel MSLN-directed protein construct HPN536. HPN536 is a T-cell-activating protein-based construct targeting MSLN-expressing tumor cells and engaging CD3ε on T cells via an albumin linker. HPN536 tethers T cells and MSLN-expressing target cells together, enabling the formation of a cytolytic synapse resulting in T cell-dependent cellular cytotoxicity (TDCC) with preclinical models demonstrating increased cell death and tumor growth inhibition[52]. A phase I trial investigating the safety and recommended phase 2 dose (RP2D) of this drug in MSLN-expressing advanced tumors, including DPM, is open but closed to recruitment (NCT03872206).
Mesothelin Antibody-Drug Conjugate (ADC). Anetumab ravtansine is an ADC comprised of an IgG1 anti-MSLN antibody conjugated to the maytansine derivative tubulin inhibitor DM4 via a reducible disulfide linker. The payload induces cell cycle arrest and apoptosis and has significant antitumor activity in preclinical xenograft mesothelioma models[53]. The initial phase 1 trial noted a promising preliminary partial response rate of 31%[54], prompting the randomized phase II ARCS-M trial examining anetumab ravtansine for the treatment of mesothelin-positive DPM. The trial randomly assigned 248 patients whose disease had progressed on prior platinum/pemetrexed with or without bevacizumab to receive anetumab ravtansine or vinorelbine. Unfortunately, the primary endpoint of PFS was no better with anetumab ravtansine than vinorelbine and there was no significant difference in OS between the groups [Table 2][55]. This large negative prospective trial highlights the importance of further refining biomarker development and mesothelin-ADCs in DPM to better characterize those most likely to benefit[57]. Another phase I/2a mesothelin-ADC clinical trial, BMS-986148, was recently published, showing an acceptable safety profile and a modest signal of clinical benefit in patients with DPM, particularly when used in combination with nivolumab (NCT02341625; Table 2)[56]. Ongoing preclinical investigations seeking to refine mesothelin-ADCs are underway[58,59].
Mesothelin-ADCs in mesothelioma
| NCT ID | Phase | Product | Target patient population | Outcomes | Reference |
| NCT02610140 | 2 | Anetumab ravtansine | 248 patients with DPM randomized 2:1 to anetumab ravtansine versus vinorelbine | Anetumab ravtansine vs. vinorelbine: PFS: 4.3 vs. 4.5 months (HR 1.22, 95%CI: 0.85-1.74); OS: 11.6 vs. 9.5 months (HR 1.07, 95%CI 0.76-1.51) | [55] |
| NCT02341625 | 1/2a | BMS-986148 +/- nivolumab | 96 patients received BMS-986148 monotherapy (n = 44 with DPM) and 30 received combination (n = 16 with DPM) | Monotherapy: DCR: 56% (n = 14) and ORR: 4% (n = 1) in DPM patients Combination: DCR: 85% (n = 11) and ORR: 23% (n = 3) in DPM patients | [56] |
Mesothelin Chimeric Antigen Receptor (CAR) T Cells: CAR-T cells are engineered to identify cancer-specific cell surface antigens and promote cell lysis via activation of an intracellular domain of the T cell receptor-CD3 complex and, in some cases, additional intracellular co-stimulatory molecules[60]. CAR-T cell products are now available for patients with several different types of refractory hematologic malignancies[61-64], and there is keen interest in exploring their applicability in solid tumors[65]. Given the overexpression of MSLN in DPM, anti-MSLN CAR-T cell constructs are in active development[65-68], with several approaches examining these agents either as single agents or in combination under investigation.
While CAR-T therapy can lead to a durable response in hematologic malignancies, several qualities of solid tumors have proven problematic, including heterogeneous antigen presentation, an inhospitable tumor microenvironment, and T-cell infiltration into a solid tumor[65,66,69]. In DPM, there have been multiple studies evaluating different MSLN-targeting CAR-T cell constructs and administration techniques (systemic vs. intrapleural; Table 3). Evaluations to date have mostly been in phase I clinical trials focused on safety and tolerability, limiting our ability to speak to definitive efficacy. Furthermore, exploration of the combination of a CD28-costimulated mesothelin CAR-T with the iCaspase-9 safety gene and pembrolizumab has shown preliminary promise with a median OS of 23.9 months, 8 patients achieving stability for 6 months or more, and 2 patients with a complete response[75]. Larger prospective studies of novel CAR-T constructs and combinations are needed to better determine the safety, efficacy, and proper patient population to deploy this exciting treatment strategy.
Anti mesothelin CAR-T cell US clinical trials in mesothelioma
| NCT ID | Phase | Product | Target patient population | Outcomes | Reference |
| CAR-T +/- chemotherapy conditioning | |||||
| NCT01355965 | 1 | Autologous mesothelin re-directed T cells | 18 patients with DPM. | 4 Patients treated with anaphylaxis, off-target toxicity | [70,71] |
| NCT02159716 | 1 | Lentiviral transduced CART-mesothelioma cells | 15 patients with DPM, ovarian ca, pancreatic ductal ca. | Cells well tolerated, expanded in blood, limited clinical activity | [72] |
| NCT03054298 | 1 | Lentiviral transduced fully human CART-mesothelioma cells | Up to 15 patients with mesothelin-expressing refractory DPM, lung cancer, and ovarian ca. | Study Ongoing | |
| NCT04577326 | 1 | M28z1XXPD1DNR: CAR T-cell with cell-intrinsic PD-1 blockade | 7 patients with DPM. | Study Ongoing | [73] |
| NCT01583686 | 1 | Anti-mesothelin CAR transduced peripheral blood lymphocytes + aldesleukin (IL-2) | 15 patients with mesothelin expressing metastatic disease. | Study Terminated for poor accrual | |
| NCT03608618 | 1 | MCY-M11: mesothelin targeting CAR-T- Intraperitoneal | 14 patients with ovarian Ca, primary peritoneal or fallopian tube ca, and peritoneal mesothelioma. | Following the treatment of 11 patients with initial feasibility and safety reported, study terminated- sponsor priority. | [74] |
| NCT05568680 | 1 | SynKIR-110: T-cell transduced with mesothelin KIR-CAR | 42 patients with ovarian Ca, primary peritoneal Ca, ovarian or fallopian tube Ca, mesotheliomas, cholangiocarcinoma | Study Ongoing | |
| NCT05451849 | 1/2 | TC-510 T cell expressing both a mesothelin-CD3ε subunit and PD-1:CD28 switch receptor | 115 patients with advanced mesothelin-expressing tumors including DPM | Study Ongoing | |
| CAR-T + Immune Checkpoint Inhibition | |||||
| NCT02414269 | 1/2 | CD28-costimulated mesothelin CAR with the Icaspase-9 safety gene (IcasM28z) + pembrolizumab (mesothelioma cohort only) | 113 patients with mesothelin expressing malignant pleural disease. | 19 DPM patients: 2 complete metabolic response on PET, 5 partial response, 4 stable disease. Study Ongoing. | [75] |
| NCT03907852 | 1/2 | Gavocabtagene autoleucel (autologous anti-mesothelin TCR fusion construct [TRuC]) with and without nivolumab or ipilimumab/nivolumab | 175 patients with advanced mesothelin-expressing cancers | Tumor regression in first 5 patients treated. Study ongoing. | [76] |
BRCA1-associated protein 1 (BAP1): BAP1 is a ubiquitin c-terminus hydrolase[14] which functions as a key tumor suppressor based on its role in both epigenetic modulation and DNA damage response[77]. Somatic and germline mutations in BAP1 are associated with multiple solid malignancies, including a significant proportion of mesotheliomas[16,78-80], melanomas (uveal and cutaneous), clear cell renal cell carcinomas, and lung adenocarcinomas[14,81]. BAP1 inactivation increases the expression of enhancer of zeste homolog 2 (EZH2; also known as histone-lysine N-methyltransferase), which has itself been implicated as an oncogenic driver in DPMs[82]. Thus, BAP1 loss may sensitize such tumors to EZH2 inhibition. With nearly two-thirds of DPM tumors having inactivation of BAP1,[16,82] it is a key biomarker under clinical development [Table 4].
BAP1 targeted therapy in mesothelioma
| NCT ID | Phase | Product | Target patient population | Outcomes | Reference |
| NCT02860286 | 2 | Tazemetostat; EZH2 inhibitor | 74 patients with previously treated BAP1 inactivated DPM | PFS: 18 weeks; OS: 36 weeks; ORR 3%; DCR: 54% at 12 weeks | [83] |
| NCT04104776 | 1/2 | CPI-0209; EZH2 inhibitor | 213 patients with advanced solid tumors and lymphomas including a cohort for BAP1 loss mesotheliomas | Study Ongoing | [84] |
| NCT03654833 | 2 | Rucaparib; PARP inhibitor | 26 patients with previously treated BAP1-deficient or BRCA1-deficient mesotheliomas | DCR at 12 weeks: 58%; manageable toxicity | [85] |
| NCT03531840 | 2 | Olaparib; PARP inhibitor | 23 patients with previously treated mesotheliomas, regardless of BAP1 status | All comers: PFS: 3.6 months and OS: 8.7 months Germline BAP1 mutation (n = 4) vs. wildtype: PFS: 2.3 vs. 4.1 months (P = 0.02); OS: 4.6 vs. 9.6 months (P = 0.004) | [86] |
| NCT05455424 | 2 | Niraparib; PARP inhibitor | 84 patients with previously treated DPM randomized to niraparib vs. active symptom control | Study Ongoing | |
| NCT04515836 | 2 | Olaparib; PARP inhibitor | 56 patients with previously treated DPM harboring mutations in homologous recombination repair | Study Ongoing | |
| NCT02535312 | 1/2 | Pemetrexed + TCR102; BER pathway inhibitor | 16 patients with previously treated DPM | PFS: 4.3 months; 2 patients with partial responses | [87] |
| NCT04940637 | 2 | niraparib + dostarlimab | 70 patients with PD-L1 +, HRd + MPM or NSCLC | Study Ongoing | [88] |
The EZH2 inhibitor tazemetostat is approved for later-line treatment of constitutively EZH2-activated tumors including epithelioid sarcomas with INI1/SMARCB1 loss[89] and follicular lymphomas harboring EZH2 mutations[90]. Given the enrichment of BAP1 inactivation in DPM, tazemetostat was explored in BAP1-inactivated DPM in a single-arm open-label phase 2 trial in 74 patients who were previously treated with platinum-based chemotherapy[83]. While the response rate was low (ORR 3% [n = 2]), the disease control rate (DCR) was 54% at 12 weeks. This trial highlights a rationally designed targeted therapeutic approach for patients with DPM. Studies are ongoing to refine the population most likely to benefit from tazemetostat, as well as investigations into novel combinations.
Other efforts have focused on leveraging the role BAP1 plays in DNA repair and attempted to create conditions of synthetic lethality by employing poly ADP-ribose polymerase (PARP) inhibitors. PARP inhibitors have known efficacy across several solid tumors including approval in patients with ovarian[91,92] or breast cancers[93] harboring a germline BRCA mutation. The non-comparative multi-arm phase 2 Mesothelioma-Stratified Therapy 1 (MiST 1) trial was a novel clinical research platform study designed to stratify patients with DPM to targeted therapies after progression on first-line chemotherapy. Arm 1 of this trial enrolled 26 patients with cytoplasmic-BAP1-deficient or BRCA1-deficient mesotheliomas after platinum-based chemotherapy[85]. Patients received oral rucaparib twice daily for 24 weeks. DCR was 58% at 12 weeks and 23% at 24 weeks; partial responses were observed in three patients (12%). Furthermore, a similar single-arm phase II trial enrolled 23 patients with refractory mesothelioma to receive the PARP inhibitor olaparib[86]. Patients in this trial were not selected by BAP1 alterations/loss (although 14 [61%] patients in the trial had BAP1 alterations). In this unselected population, olaparib had limited activity, with one (4%) partial response. In this small sample, germline BAP1 mutations were associated with decreased OS compared to wild type (4.6 vs. 9.6 months, respectively, P = 0.004).
Base excision repair (BER) is a coordinated cellular process by which damaged DNA base pairs can be excised and repaired[94]; inhibition of this pathway in a tumor with DNA damage repair deficiencies, such as BAP1 loss, could lead to synthetic lethality. A recent phase 1 trial examined the safety and activity of TCR102, a BER pathway inhibitor, in combination with chemotherapy for the treatment of multiple advanced solid tumors[87]. In the DPM cohort, 14 patients were treated with TCR102 in combination with pemetrexed resulting in two (14%) partial responses and acceptable toxicity at the RP2D, meeting the prespecified criteria to warrant further exploration. A phase 2 continuation of this trial is ongoing (NCT02535312).
Wilms Tumor 1 Protein (WT1): WT1 is a human self-antigen presented on the surface of cells, which plays a role in regulating cell proliferation and tumorigenesis. WT1 is limited to low-level expression in normal adult tissues, but the expression is enriched in several tumors, including 72%-93% of DPM[9,10,95-98], making it a provocative target for therapeutic exploitation.
A randomized phase 2 trial sought to evaluate the efficacy of a WT1 targeting peptide vaccine, Galinpepimut-S, in the adjuvant treatment of DPM. The study randomized 41 patients who had completed multimodality therapy for resectable DPM to either standard-of-care adjuvant chemotherapy and granulocyte-macrophage colony-stimulating factor (GM-CSF) with or without Galinpepimut-S. The study found the vaccine to be tolerable and a signal of improved OS (22.8 vs. 18.3 months) and PFS (10.1 vs. 7.4 months) compared to standard adjuvant chemotherapy alone; however, due to the control arm closing early for futility and the trial not being designed for a direct comparison of the two arms, a definitive efficacy signal could not be ascertained[99]. Further exploration of the WT1 vaccine is underway in a phase 1 study investigating the potential synergistic effects of Galinpepimut-S in combination with the anti-PD-L1 agent nivolumab for the treatment of patients with relapsed/refractory DPM (NCT04040231).
NF2/YAP/TAZ: Genetic alterations in neurofibromatosis type 2 (NF2) are found in approximately 40% of DPM specimens[100-102].The NF2 gene encodes moesin-ezrin-radixin-like (Merlin) tumor suppressor, and its inactivation is associated with the loss of Merlin protein expression in mesothelioma cells[103-105]. Merlin regulates the HIPPO pathway by negatively regulating transcriptional co-activators YAP and TAZ through the E3 ubiquitin ligase CRL4DCAF1; YAP and TAZ disinhibition results in an oncogenic cascade predisposing to the development of DPM[106-109]. Due to the genomic enrichment of NF2 alterations in DPM
NF2/YAP/TAZ targeted therapy in mesothelioma
| NCT ID | Phase | Product | Target patient population | Outcomes | Reference |
| NCT01870609 | 2 | Platinum pemetrexed +/- defactinib maintenance; FAK inhibitor | 344 patients with previously treated DPM randomized 1:1 after 4 cycles of chemotherapy to defactinib maintenance or placebo | Maintenance vs. placebo: PFS: 4.1 vs. 4.0 months; OS: 12.7 vs. 13.6 months (HR 1.0, 95%CI: 0.7-1.4) | [110] |
| NCT00770120 | 2 | Everolimus; mTOR inhibitor | 59 patients with DPM treated with ≤ 1 prior chemotherapy regimen | ORR: 0%; PFS 2.9 months; OS 6.3 months | [111] |
| NCT01024946 | 2 | Everolimus; mTOR inhibitor | 39 patients with previously treated DPM with NF2 loss | Closed early, given tolerability after enrolling 11 patients (6 evaluable) | |
| NCT05228015 | 1 | IK-930; TEAD inhibitor | 158 patients with previously treated advanced solid tumors | Study ongoing | |
| NCT04857372 | 1 | IAG933; YAP/TEAD inhibitor | 156 patients with previously treated DPM and other solid tumors | Study ongoing | |
| NCT04665206 | 1 | VT3989; TEAD inhibitor | 80 patients with refractory solid tumors, including DPM with NF2 loss | Study ongoing | |
| NCT03319537 | 1/2 | Pevonedistat; NEDD8 inhibitor | Monotherapy: Previously treated patients with NF2 altered DPM; Pevonedistat + platinum/pemetrexed: Treatment naïve patients with DPM | Closed to accrual |
The oral small-molecule focal adhesion kinase (FAK) TKI defactinib has been investigated in multiple solid tumor types, including ovarian, colorectal, pancreatic, and lung cancers[112]. Defactinib selectively kills Merlin-expressing cells through a FAK-Merlin synthetic lethal pathway. In a large, global, randomized phase II trial, 344 patients with DPM and with disease control after 4 cycles of first-line platinum/pemetrexed-based chemotherapy were assigned to receive either defactinib or placebo; Unfortunately, neither PFS nor OS was improved with defactinib[110]. Trials evaluating other inhibitors of this pathway are underway, including (1) Nedd8-activating enzyme (NAE) inhibitors which result in decreased formation of CRL4DCAF1(NCT03319537); and (2) YAP/TEAD inhibitors (NCT04857372).
CDKN2, p16, MTAP: Co-deletion of the CDKN2A and methylthioadenosine phosphorylase (MTAP) genes is notably enriched in 28%-49% of DPM[8,16,22,113,114]. The proximity of the CDKN2A gene on chromosome 9p21 to MTAP predisposes the loss of both genes with the loss of one allele[115,116]. CDKN2A encodes p16INK4a and p14AR, important cell cycle modulators which regulate cyclin-dependent kinases (CDKs)[117-119]. Enrichment of these alterations poses distinct mechanistic vulnerabilities under investigation: (1) Protein arginine methyltransferase 5 (PRMT5); and (2) Cyclin-dependent kinase (CDK) inhibition [Table 6].
CDKN2/p16/MTAP targeted therapy in mesothelioma
| NCT ID | Phase | Product | Target Patient Population | Outcomes | Reference |
| NCT03654833 | 2 | Abemaciclib; CDK4/6 inhibitor | 27 eligible patients with previously treated DPM with IHC noting p16ink4A deficiency | DCR at 12 weeks: 54%; PFS: 128 days; OS: 217 days | [120] |
| NCT05538572 | 1 | PRT3645; CDK4/6 inhibitor | 51 patients with previously treated advanced solid tumors | Study ongoing | |
| NCT05245500 | 1/2 | MRTX1719; PRMT5-MTA inhibitor | 339 patients with previously treated advanced MTAP-deleted solid tumors | Study ongoing | |
| NCT05275478 | 1 | TNG908; PRMT5 inhibitor | 170 patients with previously treated MTAP-deleted solid tumors | Study ongoing | |
| NCT04794699 | 1 | IDE397; MAT2A Inhibitor | 382 patients with previously treated MTAP-deleted advanced solid tumors | Study ongoing |
In vitro, PRMT5 inhibition has been evaluated as a potential therapy against MTAP-deficient cancers, including DPM[121]. Early phase clinical trials of the safety and possible roles of PRMT5 inhibitors in solid tumors, including DPM, are currently underway (NCT05245500, NCT05275478, NCT04794699). Direct CDK4/6 inhibitors have synthetic lethality in DPM[122,123] and are under active investigation. In the single-arm, phase 2, MiST 2 trial, 26 patients with p16INK4A-deficient DPM whose disease had progressed after platinum-based chemotherapy received the oral CDK4/6 inhibitor, abemaciclib. The study met its primary endpoint, with a DCR of 54% at 12 weeks, tumor volume reductions in 80% of evaluable patients, and four patients who achieved a partial response[120]. These results are encouraging evidence of possible antitumor effects, but a larger randomized trial and further refinement of possible biomarkers are needed to determine any possible place in our current clinical practice[124,125].
V-domain Ig suppressor T cell activation (VISTA): VISTA is a negative immune checkpoint regulator of myeloid and T cell function with high levels of expression in DPM (85%)[17]. In vivo studies suggest anti-VISTA antibodies have promising antitumor activity[16,17,126]. With the integration of 1L immunotherapy into the treatment paradigms for DPM[18], exploration of later-line treatment options to improve response and/or rechallenge to immunotherapy is needed. A phase 1 study of CA-170 (small molecule inhibitor of PD-L1 and VISTA)[127] in patients with previously treated advanced solid tumors and lymphomas exhibited an acceptable toxicity profile[128] and is currently under development. The VISTA inhibitor CI-8993 is currently under investigation in a phase I study evaluating the safety and activity of this antibody in patients with previously treated advanced solid tumors (NCT04475523).
Argininosuccinate synthetase 1 (ASS1): ASS1 is a key enzyme in the urea cycle required for the formation of arginine, and ASS1-deficiency has been implicated in tumorigenesis by supporting cellular proliferation and pyrimidine synthesis[129,130]. Certain solid tumors, including nearly two-thirds of DPM, have inherent enrichment for ASS1-deficiency[131] and mechanistically lend themselves to therapeutic exploitation with arginine deprivation therapy by pegylated arginine deaminase (ADI-PEG 20). The phase 2 study of ADI-PEG 20 in combination with cisplatin/pemetrexed in 32 patients with previously untreated ASS1-deficient DPM showed promising clinical benefit (DCR: 93.5%, PFS: 5.6 months, OS: 10.1 months)[132]. The trial expanded into the randomized phase 3 ATOMIC-MESO trial (NCT02709512) comparing cisplatin/pemetrexed with or without ADI-PEG 20 with a recent press release indicating it has met the prespecified endpoint with a median OS of 9.3 vs. 7.7 months (HR: 0.71; 95%CI: 0.55-0.93) and PFS of 6.1 vs. 5.6 months (HR: 0.65; 95%CI: 0.47-0.90); there is a current plan to submit for regulatory consideration[133]. This landmark positive trial marks a major step forward in our efforts to integrate targeted agents into the treatment of DPM.
CONCLUSION
With a growing understanding of the molecular underpinnings of DPM, there has been a multitude of rationally designed clinical trials looking to exploit potential therapeutic vulnerabilities. Newer generations of agents, including CAR-T therapies targeting mesothelin and arginine-deprivation therapies for ASS1 deficient mesotheliomas, hold particular promise, and aim to overcome the historically poor response rates in targeted therapies for mesothelioma.
The preponderance of disappointing trial results described here, however, highlights the struggle to translate promising preclinical data into patient care. To propel the field forward, we must continue to collaborate to establish preclinical models that faithfully recapitulate DPM biology for in vivo testing[134] and strive to better refine biomarkers and patient selection criteria for trials of targeted therapy in DPM. Investigation of several promising preclinical targets (e.g., microRNAs) is underway but has not yet been translated into clinical investigation[135]; Future trials need to incorporate comprehensive pathologic, genomic, and expression level data of enrolled patients to better understand those who benefit from a treatment and refine future trial designs[136]. To accomplish this will require building platforms for close iterative collaboration between medical and surgical oncologists, pathologists, and laboratory-based genomic and pharmacologic scientists; this investment is critical to improving therapeutic options for patients with DPM.
DECLARATIONS
Authors’ contributionsThe following authors made substantial contributions to the conception and design of the work, literature review, technical support, and writing of the work: Offin M, Fitzgerald B, Zauderer MG, Doroshow D
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis research was supported in part by the NIH/NCI (P30 CA008748).
Conflicts of interestMichael Offin has consulted with Novartis, Jazz, and PharmaMar regarding oncology drug development. Michael Offin has received an honorarium from Targeted Oncology, OncLive, and the American Society for Radiation Oncology.
Bailey Fitzgerald has no relevant conflicts to declare.
Marjorie G. Zauderer has received consulting fees from Ikena, Takeda, GlaxoSmithKline, Aldeyra Therapeutics, and Novocure and honoraria for CME content from PER, Medscape, Research to Practice, Medical Learning Institute and OncLive. Memorial Sloan Kettering receives research funding from the Department of Defense, the National Institutes of Health, Precog, GlaxoSmithKline, Epizyme, Polaris, Sellas Life Sciences, Bristol Myers Squibb, Millenium/Takeda, Curis, and Atara for research conducted by Marjorie G. Zauderer. Marjorie G. Zauderer serves as Chair of the Board of Directors of the Mesothelioma Applied Research Foundation, an uncompensated position.
Deborah Doroshow has received consulting fees from AstraZeneca and Mirati Therapeutics and honoraria from PER, OncLive, and Targeted Oncology.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Van Gerwen M, Alpert N, Wolf A, et al. Prognostic factors of survival in patients with malignant pleural mesothelioma: an analysis of the National Cancer Database. Carcinogenesis 2019;40:529-36.
2. Alpert N, van Gerwen M, Taioli E. Epidemiology of mesothelioma in the 21st century in Europe and the United States, 40 years after restricted/banned asbestos use. Transl Lung Cancer Res 2020;9:S28-38.
3. Tsao AS, Wistuba I, Roth JA, Kindler HL. Malignant pleural mesothelioma. J Clin Oncol 2009;27:2081-90.
4. Teta MJ, Mink PJ, Lau E, Sceurman BK, Foster ED. US mesothelioma patterns 1973-2002: indicators of change and insights into background rates. Eur J Cancer Prev 2008;17:525-34.
5. Serveillance research program NCI. SEER*explorer: an interactive website for SEER cancer statistics. Available from: https://seer.cancer.gov/statistics-network/explorer/ [Last accessed on 31 May 2023].
6. Rimner A, Zauderer MG, Gomez DR, et al. Phase II study of hemithoracic intensity-modulated pleural radiation therapy (IMPRINT) as part of lung-sparing multimodality therapy in patients with malignant pleural mesothelioma. J Clin Oncol 2016;34:2761-8.
7. Weder W, Opitz I. Multimodality therapy for malignant pleural mesothelioma. Ann Cardiothorac Surg 2012;1:502-7.
8. Bueno R, Stawiski EW, Goldstein LD, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet 2016;48:407-16.
9. Amin KM, Litzky LA, Smythe WR, et al. Wilms’ tumor 1 susceptibility (WT1) gene products are selectively expressed in malignant mesothelioma. Am J Pathol 1995;146:344-56.
10. Oji Y, Ogawa H, Tamaki H, et al. Expression of the Wilms’ tumor gene WT1 in solid tumors and its involvement in tumor cell growth. Jpn J Cancer Res 1999;90:194-204.
12. Servais EL, Colovos C, Rodriguez L, et al. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clin Cancer Res 2012;18:2478-89.
13. Arzt L, Quehenberger F, Halbwedl I, Mairinger T, Popper HH. BAP1 protein is a progression factor in malignant pleural mesothelioma. Pathol Oncol Res 2014;20:145-51.
14. Cheung M, Testa JR. BAP1, a tumor suppressor gene driving malignant mesothelioma. Transl Lung Cancer Res 2017;6:270-8.
15. Yoshikawa Y, Sato A, Tsujimura T, et al. Frequent inactivation of the BAP1 gene in epithelioid-type malignant mesothelioma. Cancer Sci 2012;103:868-74.
16. Hmeljak J, Sanchez-Vega F, Hoadley KA, et al. Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discov 2018;8:1548-65.
17. Muller S, Victoria Lai W, Adusumilli PS, et al. V-domain Ig-containing suppressor of T-cell activation (VISTA), a potentially targetable immune checkpoint molecule, is highly expressed in epithelioid malignant pleural mesothelioma. Mod Pathol 2020;33:303-11.
18. Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021;397:375-86.
19. Aggarwal C, Albelda SM. Molecular characterization of malignant mesothelioma: time for new targets? Cancer Discov 2018;8:1508-10.
20. Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44.
21. Offin M, Yang SR, Egger J, et al. Molecular characterization of peritoneal mesotheliomas. J Thorac Oncol 2022;17:455-60.
22. Zauderer MG, Martin A, Egger J, et al. The use of a next-generation sequencing-derived machine-learning risk-prediction model (OncoCast-MPM) for malignant pleural mesothelioma: a retrospective study. Lancet Digit Health 2021;3:e565-76.
23. Hung YP, Dong F, Watkins JC, et al. Identification of ALK rearrangements in malignant peritoneal mesothelioma. JAMA Oncol 2018;4:235-8.
24. Mian I, Abdullaev Z, Morrow B, et al. Anaplastic lymphoma kinase gene rearrangement in children and young adults with mesothelioma. J Thorac Oncol 2020;15:457-61.
25. Cornelissen R, Dubbink HJ, von der Thüsen JH. ALK in mesothelioma: to fish or not to fish? J Thorac Oncol 2020;15:e168-9.
26. Leal JL, Peters G, Szaumkessel M, et al. NTRK and ALK rearrangements in malignant pleural mesothelioma, pulmonary neuroendocrine tumours and non-small cell lung cancer. Lung Cancer 2020;146:154-9.
27. Chen Y, Chen B, Zhu X, Zhong J. A patient with malignant pleural mesothelioma carrying BRAF V600E mutation responding to vemurafenib. Lung Cancer 2018;116:96-8.
28. Rüschoff JH, Gradhand E, Kahraman A, et al. STRN-ALK rearranged malignant peritoneal mesothelioma with dramatic response following ceritinib treatment. JCO Precis Oncol 2019:3.
30. National Comprehensive Cancer Network. Malignant pleural mesothelioma (version 1.2023). Available from: https://www.nccn.org/professionals/physician_gls/pdf/mpm.pdf [Last accessed on 31 May 2023].
31. Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405-14.
33. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 2018;15:325-40.
34. Pinto C, Zucali PA, Pagano M, et al. Gemcitabine with or without ramucirumab as second-line treatment for malignant pleural mesothelioma (RAMES): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2021;22:1438-47.
36. Hiltbrunner S, Fleischmann Z, Sokol ES, Zoche M, Felley-Bosco E, Curioni-Fontecedro A. Genomic landscape of pleural and peritoneal mesothelioma tumours. Br J Cancer 2022;127:1997-2005.
37. Raghav K, Liu S, Overman MJ, et al. Efficacy, safety, and biomarker analysis of combined PD-L1 (Atezolizumab) and VEGF (Bevacizumab) blockade in advanced malignant peritoneal mesothelioma. Cancer Discov 2021;11:2738-47.
38. Tsao AS, Miao J, Wistuba II, et al. SWOG S0905: a randomized phase II study of cediranib versus placebo in combination with cisplatin and pemetrexed in chemonaive patients with malignant pleural mesothelioma. J Clin Oncol 2018;36:8514-8514.
39. Tsao AS, Miao J, Wistuba II, et al. Phase II trial of cediranib in combination with cisplatin and pemetrexed in chemotherapy-naïve patients with unresectable malignant pleural mesothelioma (SWOG S0905). J Clin Oncol 2019;37:2537-47.
40. Campbell NP, Kunnavakkam R, Leighl N, et al. Cediranib in patients with malignant mesothelioma: a phase II trial of the University of Chicago Phase II Consortium. Lung Cancer 2012;78:76-80.
41. Scagliotti GV, Gaafar R, Nowak AK, et al. Nintedanib in combination with pemetrexed and cisplatin for chemotherapy-naive patients with advanced malignant pleural mesothelioma (LUME-Meso): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet Respir Med 2019;7:569-80.
42. Wozniak AJ, Schneider BJ, Kalemkerian GP, et al. A phase II trial of nintedanib in recurrent malignant pleural mesothelioma (MPM). J Clin Oncol 2019;37:e20061-e20061.
43. Pastan I, Hassan R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res 2014;74:2907-12.
44. Bera TK, Pastan I. Mesothelin is not required for normal mouse development or reproduction. Mol Cell Biol 2000;20:2902-6.
45. Hu ZI, Ghafoor A, Sengupta M, Hassan R. Malignant mesothelioma: advances in immune checkpoint inhibitor and mesothelin-targeted therapies. Cancer 2021;127:1010-20.
46. Hassan R, Kindler HL, Jahan T, et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin Cancer Res 2014;20:5927-36.
47. Leshem Y, King EM, Mazor R, Reiter Y, Pastan I. SS1P immunotoxin induces markers of immunogenic cell death and enhances the effect of the CTLA-4 blockade in AE17M mouse mesothelioma tumors. Toxins 2018;10:470.
48. Hassan R, Sharon E, Thomas A, et al. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer 2014;120:3311-9.
49. Hassan R, Miller AC, Sharon E, et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med 2013;5:208ra147.
50. Bauss F, Lechmann M, Krippendorff BF, et al. Characterization of a re-engineered, mesothelin-targeted Pseudomonas exotoxin fusion protein for lung cancer therapy. Mol Oncol 2016;10:1317-29.
51. Hassan R, Alewine C, Mian I, et al. Phase 1 study of the immunotoxin LMB-100 in patients with mesothelioma and other solid tumors expressing mesothelin. Cancer 2020;126:4936-47.
52. Molloy ME, Austin RJ, Lemon BD, et al. Preclinical Characterization of HPN536, a trispecific, T-cell-activating protein construct for the treatment of mesothelin-expressing solid tumors. Clin Cancer Res 2021;27:1452-62.
53. Golfier S, Kopitz C, Kahnert A, et al. Anetumab ravtansine: a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol Cancer Ther 2014;13:1537-48.
54. Hassan R, Blumenschein GR Jr, Moore KN, et al. First-in-human, multicenter, phase I dose-escalation and expansion study of anti-mesothelin antibody-drug conjugate anetumab ravtansine in advanced or metastatic solid tumors. J Clin Oncol 2020;38:1824-35.
55. Kindler HL, Novello S, Bearz A, et al. Anetumab ravtansine versus vinorelbine in patients with relapsed, mesothelin-positive malignant pleural mesothelioma (ARCS-M): a randomised, open-label phase 2 trial. Lancet Oncol 2022;23:540-52.
56. Rottey S, Clarke J, Aung K, et al. Phase I/IIa trial of BMS-986148, an anti-mesothelin antibody-drug conjugate, alone or in combination with nivolumab in patients with advanced solid tumors. Clin Cancer Res 2022;28:95-105.
57. Zauderer MG, Offin M. ARCS-M: forging progress from this negative trial in malignant pleural mesothelioma. Lancet Oncol 2022;23:445-6.
58. Hsu HJ, Tung CP, Yu CM, et al. Eradicating mesothelin-positive human gastric and pancreatic tumors in xenograft models with optimized anti-mesothelin antibody-drug conjugates from synthetic antibody libraries. Sci Rep 2021;11:15430.
59. Terwisscha van Scheltinga AG, Ogasawara A, Pacheco G, et al. Preclinical efficacy of an antibody-drug conjugate targeting mesothelin correlates with quantitative 89Zr-ImmunoPET. Mol Cancer Ther 2017;16:134-42.
60. Jayaraman J, Mellody MP, Hou AJ, et al. CAR-T design: elements and their synergistic function. EBioMedicine 2020;58:102931.
61. Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra25.
62. Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540-9.
63. Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509-18.
64. Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med 2019;380:1726-37.
65. Morello A, Sadelain M, Adusumilli PS. Mesothelin-targeted CARs: driving T cells to solid tumors. Cancer Discov 2016;6:133-46.
66. Chintala NK, Restle D, Quach H, et al. CAR T-cell therapy for pleural mesothelioma: rationale, preclinical development, and clinical trials. Lung Cancer 2021;157:48-59.
67. Adusumilli PS, Cherkassky L, Villena-Vargas J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med 2014;6:261ra151.
68. Tokatlian T, Asuelime GE, Mock JY, et al. Mesothelin-specific CAR-T cell therapy that incorporates an HLA-gated safety mechanism selectively kills tumor cells. J Immunother Cancer 2022;10:e003826.
69. Newick K, Moon E, Albelda SM. Chimeric antigen receptor T-cell therapy for solid tumors. Mol Ther Oncolytics 2016;3:16006.
70. Beatty GL, Haas AR, Maus MV, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res 2014;2:112-20.
71. Maus MV, Haas AR, Beatty GL, et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res 2013;1:26-31.
72. Haas AR, Tanyi JL, O’Hara MH, et al. Phase I study of lentiviral-transduced chimeric antigen receptor-modified t cells recognizing mesothelin in advanced solid cancers. Mol Ther 2019;27:1919-29.
73. Kiesgen S, Linot C, Quach HT, et al. Abstract LB-378: regional delivery of clinical-grade mesothelin-targeted CAR T cells with cell-intrinsic PD-1 checkpoint blockade: Translation to a phase I trial. Cancer Res 2020;80:LB-378.
74. Annunziata CM, Ghobadi A, Pennella EJ, Vanas J, Powell C, Pavelova M et al. Feasibility and preliminary safety and efficacy of first-in-human intraperitoneal delivery of MCY-M11, anti-human-mesothelin CAR mRNA transfected into peripheral blood mononuclear cells, for ovarian cancer and malignant peritoneal mesothelioma. J Clin Oncol 2020;38:3014.
75. Adusumilli PS, Zauderer MG, Rivière I, et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discov 2021;11:2748-63.
76. TCR Therapeutics. TCR2 therapeutics announces recist responses with first TC-210 dose tested in advanced mesothelin-expressing solid tumors. Available from: https://investors.tcr2.com/news-releases/news-release-details/tcr2-therapeutics-announces-recist-responses-first-tc-210-dose [Last accessed on 31 May 2023].
77. Obacz J, Yung H, Shamseddin M, et al. Biological basis for novel mesothelioma therapies. Br J Cancer 2021;125:1039-55.
78. Baumann F, Flores E, Napolitano A, et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis 2015;36:76-81.
79. Panou V, Gadiraju M, Wolin A, et al. Frequency of germline mutations in cancer susceptibility genes in malignant mesothelioma. J Clin Oncol 2018;36:2863-71.
80. Bott M, Brevet M, Taylor BS, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet 2011;43:668-72.
81. Testa JR, Cheung M, Pei J, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet 2011;43:1022-5.
82. LaFave LM, Béguelin W, Koche R, et al. Loss of BAP1 function leads to EZH2-dependent transformation. Nat Med 2015;21:1344-9.
83. Zauderer MG, Szlosarek PW, Le Moulec S, et al. EZH2 inhibitor tazemetostat in patients with relapsed or refractory, BAP1-inactivated malignant pleural mesothelioma: a multicentre, open-label, phase 2 study. Lancet Oncol 2022;23:758-67.
84. Lakhani NJ, Gutierrez M, Duska LR, et al. Phase 1/2 first-in-human (FIH) study of CPI-0209, a novel small molecule inhibitor of enhancer of zeste homolog 2 (EZH2) in patients with advanced tumors. JCO 2021;39:3104-3104.
85. Fennell DA, King A, Mohammed S, et al. Rucaparib in patients with BAP1-deficient or BRCA1-deficient mesothelioma (MiST1): an open-label, single-arm, phase 2a clinical trial. Lancet Respir Med 2021;9:593-600.
86. Ghafoor A, Mian I, Wagner C, et al. Phase 2 study of olaparib in malignant mesothelioma and correlation of efficacy with germline or somatic mutations in BAP1 gene. JTO Clin Res Rep 2021;2:100231.
87. Koczywas M. Phase I study of TRC102 in combination with cisplatin and pemetrexed in patients with advanced solid tumors/Phase II study of TRC102 with pemetrexed in patients with mesothelioma refractory to pemetrexed and cisplatin or carboplatin. J Clin Oncol 2020;38:9055.
88. Passiglia F, Bironzo P, Righi L, et al. A prospective phase II single-arm study of niraparib plus dostarlimab in patients with advanced non-small-cell lung cancer and/or malignant pleural mesothelioma, positive for PD-L1 expression and germline or somatic mutations in the DNA repair genes: rationale and study design. Clin Lung Cancer 2021;22:e63-6.
89. Gounder M, Schöffski P, Jones RL, et al. Tazemetostat in advanced epithelioid sarcoma with loss of INI1/SMARCB1: an international, open-label, phase 2 basket study. Lancet Oncol 2020;21:1423-32.
90. Morschhauser F, Tilly H, Chaidos A, et al. Tazemetostat for patients with relapsed or refractory follicular lymphoma: an open-label, single-arm, multicentre, phase 2 trial. Lancet Oncol 2020;21:1433-42.
91. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2018;379:2495-505.
92. Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1274-84.
93. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 2017;377:523-33.
94. Carter RJ, Parsons JL. Base excision repair, a pathway regulated by posttranslational modifications. Mol Cell Biol 2016;36:1426-37.
95. Mundlos S, Pelletier J, Darveau A, Bachmann M, Winterpacht A, Zabel B. Nuclear localization of the protein encoded by the Wilms’ tumor gene WT1 in embryonic and adult tissues. Development 1993;119:1329-41.
96. Foster MR, Johnson JE, Olson SJ, Allred DC. Immunohistochemical analysis of nuclear versus cytoplasmic staining of WT1 in malignant mesotheliomas and primary pulmonary adenocarcinomas. Arch Pathol Lab Med 2001;125:1316-20.
97. Oates J, Edwards C. HBME-1, MOC-31, WT1 and calretinin: an assessment of recently described markers for mesothelioma and adenocarcinoma. Histopathology 2000;36:341-7.
98. Ordóñez NG. The immunohistochemical diagnosis of mesothelioma: a comparative study of epithelioid mesothelioma and lung adenocarcinoma. Am J Surg Pathol 2003;27:1031-51.
99. Zauderer MG, Tsao AS, Dao T, et al. A randomized phase II trial of adjuvant galinpepimut-S, WT-1 analogue peptide vaccine, after multimodality therapy for patients with malignant pleural mesothelioma. Clin Cancer Res 2017;23:7483-9.
100. Sekido Y, Pass HI, Bader S, Mew DJ, Christman MF, Gazdar AF et al. Neurofibromatosis type 2 (NF2) gene is somatically mutated in mesothelioma but not in lung cancer. Cancer Res 1995;55:1227-31.
101. Bianchi AB, Mitsunaga SI, Cheng JQ, et al. High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc Natl Acad Sci USA 1995;92:10854-8.
102. Ladanyi M, Zauderer MG, Krug LM, et al. New strategies in pleural mesothelioma: BAP1 and NF2 as novel targets for therapeutic development and risk assessment. Clin Cancer Res 2012;18:4485-90.
103. Okada M, Kijima T, Aoe K, et al. Clinical efficacy and safety of nivolumab: results of a multicenter, open-label, single-arm, japanese phase II study in Malignant pleural mesothelioma (MERIT). Clin Cancer Res 2019;25:5485-92.
104. Poulikakos PI, Xiao GH, Gallagher R, Jablonski S, Jhanwar SC, Testa JR. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene 2006;25:5960-8.
105. Shapiro IM, Kolev VN, Vidal CM, et al. Merlin deficiency predicts FAK inhibitor sensitivity: a synthetic lethal relationship. Sci Transl Med 2014;6:237ra68.
106. Miyanaga A, Masuda M, Tsuta K, et al. Hippo pathway gene mutations in malignant mesothelioma: revealed by RNA and targeted exon sequencing. J Thorac Oncol 2015;10:844-51.
107. Sato T, Sekido Y. NF2/Merlin inactivation and potential therapeutic targets in mesothelioma. Int J Mol Sci 2018;19:988.
108. Zhang WQ, Dai YY, Hsu PC, et al. Targeting YAP in malignant pleural mesothelioma. J Cell Mol Med 2017;21:2663-76.
109. Cooper J, Xu Q, Zhou L, et al. Combined inhibition of NEDD8-activating enzyme and mTOR suppresses NF2 loss-driven tumorigenesis. Mol Cancer Ther 2017;16:1693-704.
110. Fennell DA, Baas P, Taylor P, et al. Maintenance defactinib versus placebo after first-line chemotherapy in patients with merlin-stratified pleural mesothelioma: COMMAND-A double-blind, randomized, phase II study. J Clin Oncol 2019;37:790-8.
111. Ou SH, Moon J, Garland LL, et al. SWOG S0722: phase II study of mTOR inhibitor everolimus (RAD001) in advanced malignant pleural mesothelioma (MPM). J Thorac Oncol 2015;10:387-91.
112. Kulkarni NS, Gupta V. Repurposing therapeutics for malignant pleural mesothelioma (MPM) - Updates on clinical translations and future outlook. Life Sci 2022;304:120716.
113. Cheng YY, Yuen ML, Rath EM, et al. CDKN2A and MTAP are useful biomarkers detectable by droplet digital PCR in malignant pleural mesothelioma: a potential alternative method in diagnosis compared to fluorescence in situ hybridisation. Front Oncol 2020;10:579327.
114. Ladanyi M. Implications of P16/CDKN2A deletion in pleural mesotheliomas. Lung Cancer 2005;49 Suppl 1:S95-8.
115. Olopade OI, Pomykala HM, Hagos F, et al. Construction of a 2.8-megabase yeast artificial chromosome contig and cloning of the human methylthioadenosine phosphorylase gene from the tumor suppressor region on 9p21. Proc Natl Acad Sci USA 1995;92:6489-93.
116. Hustinx SR, Leoni LM, Yeo CJ, et al. Concordant loss of MTAP and p16/CDKN2A expression in pancreatic intraepithelial neoplasia: evidence of homozygous deletion in a noninvasive precursor lesion. Mod Pathol 2005;18:959-63.
117. Perera ND, Mansfield AS. The evolving therapeutic landscape for malignant pleural mesothelioma. Curr Oncol Rep 2022;24:1413-23.
118. Guo G, Chmielecki J, Goparaju C, et al. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res 2015;75:264-9.
120. Fennell DA, King A, Mohammed S, et al. Abemaciclib in patients with p16ink4A-deficient mesothelioma (MiST2): a single-arm, open-label, phase 2 trial. Lancet Oncol 2022;23:374-81.
121. Barbarino M, Cesari D, Bottaro M, et al. PRMT5 silencing selectively affects MTAP-deleted mesothelioma: In vitro evidence of a novel promising approach. J Cell Mol Med 2020;24:5565-77.
122. Bonelli MA, Digiacomo G, Fumarola C, et al. Combined Inhibition of CDK4/6 and PI3K/AKT/mTOR pathways induces a synergistic anti-tumor effect in malignant pleural mesothelioma cells. Neoplasia 2017;19:637-48.
123. Frizelle SP, Grim J, Zhou J, et al. Re-expression of p16INK4a in mesothelioma cells results in cell cycle arrest, cell death, tumor suppression and tumor regression. Oncogene 1998;16:3087-95.
124. Nardone V, Porta C, Giannicola R, Correale P, Mutti L. Abemaciclib for malignant pleural mesothelioma. Lancet Oncol 2022;23:e237.
125. Fennell DA, Nusrat N. Abemaciclib for malignant pleural mesothelioma - Authors’ reply. Lancet Oncol 2022;23:e238.
126. Chung YS, Kim M, Cha YJ, Kim KA, Shim HS. Expression of V-set immunoregulatory receptor in malignant mesothelioma. Mod Pathol 2020;33:263-70.
127. Sasikumar PG, Sudarshan NS, Adurthi S, et al. PD-1 derived CA-170 is an oral immune checkpoint inhibitor that exhibits preclinical anti-tumor efficacy. Commun Biol 2021;4:699.
128. Bang Y, Sosman J, Daud A, et al. Phase 1 study of CA-170, a first-in-class, orally available, small molecule immune checkpoint inhibitor (ICI) dually targeting VISTA and PD-L1, in patients with advanced solid tumors or lymphomas. J Immunother Cancer 2018;6:114.
129. Rabinovich S, Adler L, Yizhak K, et al. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature 2015;527:379-83.
130. Delage B, Fennell DA, Nicholson L, et al. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int J Cancer 2010;126:2762-72.
131. Szlosarek PW, Klabatsa A, Pallaska A, et al. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin Cancer Res 2006;12:7126-31.
132. Szlosarek PW, Phillips MM, Pavlyk I, et al. Expansion phase 1 study of pegargiminase plus pemetrexed and cisplatin in patients with argininosuccinate synthetase 1-deficient mesothelioma: safety, efficacy, and resistance mechanisms. JTO Clin Res Rep 2020;1:100093.
133. Polaris. Polaris group announces positive top-line results from phase 2/3 atomic study in patients with malignant pleural mesothelioma to assess adi-peg 20 with pemetrexed and cisplatin. Available from: https://polarispharma.com/2022/09/21/20220921001/?lang=en [Last accessed on 31 May 2023].
134. Offin M, Sauter JL, Tischfield SE, et al. Genomic and transcriptomic analysis of a diffuse pleural mesothelioma patient-derived xenograft library. Genome Med 2022;14:127.
135. El Bezawy R, Percio S, Ciniselli CM, et al. miR-550a-3p is a prognostic biomarker and exerts tumor-suppressive functions by targeting HSP90AA1 in diffuse malignant peritoneal mesothelioma. Cancer Gene Ther 2022;29:1394-404.
136. Tsao AS, Lindwasser OW, Adjei AA, et al. Current and future management of malignant mesothelioma: a consensus report from the national cancer institute thoracic malignancy steering committee, international association for the study of lung cancer, and mesothelioma applied research foundation. J Thorac Oncol 2018;13:1655-67.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Offin M, Fitzgerald B, Zauderer MG, Doroshow D. The past, present, and future of targeted therapeutic approaches in patients with diffuse pleural mesotheliomas. J Cancer Metastasis Treat 2023;9:21. http://dx.doi.org/10.20517/2394-4722.2022.140
AMA Style
Offin M, Fitzgerald B, Zauderer MG, Doroshow D. The past, present, and future of targeted therapeutic approaches in patients with diffuse pleural mesotheliomas. Journal of Cancer Metastasis and Treatment. 2023; 9: 21. http://dx.doi.org/10.20517/2394-4722.2022.140
Chicago/Turabian Style
Offin, Michael, Bailey Fitzgerald, Marjorie G. Zauderer, Deborah Doroshow. 2023. "The past, present, and future of targeted therapeutic approaches in patients with diffuse pleural mesotheliomas" Journal of Cancer Metastasis and Treatment. 9: 21. http://dx.doi.org/10.20517/2394-4722.2022.140
ACS Style
Offin, M.; Fitzgerald B.; Zauderer MG.; Doroshow D. The past, present, and future of targeted therapeutic approaches in patients with diffuse pleural mesotheliomas. J. Cancer. Metastasis. Treat. 2023, 9, 21. http://dx.doi.org/10.20517/2394-4722.2022.140
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 4 clicks
Cite This Article 4 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.