Photodynamic effect of indocyanine green and its application to hepatocellular carcinoma treatment
Abstract
Hepatocellular carcinoma (HCC) stands as the primary cause of liver cancers, with limited treatment options outside of surgical resection or transplant. Photodynamic therapy (PDT) using indocyanine green (ICG) as a photosensitizer offers a promising therapeutic option for HCC. ICG PDT has demonstrated efficacy in vitro and
Keywords
INTRODUCTION
Liver cancer is a significant global health concern, with estimates suggesting that there will be over a million cases by 2025[1]. The predominant type of liver cancer is hepatocellular carcinoma (HCC), which affects up to 85% of patients with cirrhosis and is now the fifth most prevalent cancer worldwide[2].
HCC is a highly aggressive cancer that often presents in advanced stages due to the absence of clinical symptoms in its early stages[3]. In such advanced stages, it was found that radio or chemotherapy was not suitable and treatment with molecular target drugs, such as Sorafenib, was recommended. Although this led to a significant increase in overall survival, only a minority of HCC patients derive benefits from pharmacological intervention. Many patients encountered complications with high drug-related significant adverse effects and economic cost[4].
Studies found that surgery and liver transplantation (LT) remain the most effective treatments for patients with cirrhosis, as well as for non-cirrhotic patients[5-9]. Furthermore, despite the various treatment options available for patients with HCC, only orthotopic liver transplantation (OLT) or surgical resection is curative[3]. However, there are clear risks of HCC recurrence due to surgical complications such as postoperative liver failure and infected biloma[10,11]. To reduce these risks, accurate resection is crucial to identify liver segments before parenchymal transection[12]. Previous techniques, such as injection of indo carmine, have fallen short in clearly demarcating segment boundaries, particularly in cirrhotic patients. Thus, refinements and advancements in surgical techniques, such as ICG combined with near-infrared (NIR) light photodynamic therapy (PDT), were developed to overcome such limitations.
In recent years, PDT has seen notable progress with the development of a navigation system designed to identify HCC lesions during surgery by using an ICG accumulation in HCC cells for anticancer treatment[13-19]. PDT has shown significant advancements in the 21st century. While it had primarily been tested in clinical settings for oncology to treat various cancers, its application in hepatobiliary diseases remained relatively unexplored by the end of the 20th century[20-25]. Previous studies developed a PDoX model to increase the microvessel densities of subcutaneous tumor cells, which are fast-growing, metastatic, and infiltrate neighboring tissues and organs. It was found that when this model was combined with indocyanine green ICG phototherapy, highly effective and realistic responses to phototherapeutic stimuli were reproduced[24,26].
Studies found that ICG, in particular, has the greatest potential for PDT in HCC treatment[27]. ICG is a near-infrared dye that is excited by an external light and activated by photosensitizers (PSs) and accumulates in target cells, including HCC cells[13,14,20]. Increased doses of photosensitizers, such as NIR laser, are used for PDT-induced apoptosis, which induces direct tissue injury and cell apoptosis[20]. When combined with NIR laser for PDT, it is a safe, minimally invasive antitumor treatment with a low incidence of adverse reactions, less than 0.01%[14].
Initially used in HCC surgery in 2009, ICG’s application as an infrared fluorescent dye has advanced and expanded to other fields due to the rapid development of molecular imaging technology[26].
In addition to its other uses, NIR PDT has also been employed to treat several cancer models, including HCC[27]. The application of ICG and NIR for non-invasively targeting deep-seated PDOX HCC was found to be successful under strict laser safety guidelines. Extensive assessment of the clinical feasibility of ICG-based phototherapy in this model underscores its potential[24,27]. However, the unique invasiveness and vascularity displayed by HCC, along with its surrounding ICG-absorptive tissue layers, present distinct challenges not encountered in ectopic xenografts (PDOX). Therefore, it is recommended that further studies be conducted to determine optimal doses of ICG for human applications, considering both clinical efficacy and duration of phototherapy. Moreover, additional research is warranted to confirm whether the majority of patient-derived tumors exhibit a preference for ICG uptake and respond favorably to phototherapy, thereby targeting the tumoricidal process. This is particularly important given that PDOX mice originated from biopsy samples of a single patient, and previous in vivo studies may not fully reflect every clinical scenario[24].
Clinical considerations for ICG photodynamic therapy
ICG serves as a photosensitizer capable of inducing immunogenic cell death (ICD) in cancer cells. Approved by the US Food and Drug Administration (FDA) for cancer treatment, it is utilized in PDT and photothermal therapy (PTT) under near-infrared light (808 nm) irradiation[28]. Fluorescent imaging using ICG has been widely used in cancer-related research, with PDT emerging as a preferred non-invasive treatment option due to its reduced toxicity and ability to improve antitumor immune responses[29]. However, despite its promising approach in clinical settings, its utilization has been limited due to light delivery, the effectiveness of photosensitizers influenced by various factors, and low O2 generation in PDT[30].
Light delivery
The drawback in light delivery to activate photosensitizers led to studies and advancements in light delivery technologies, particularly fiber optics. These developments aim to improve clinical strategies for reaching deep tissues during irradiation[31]. Recently, nanomaterials have gained particular attention as a potential solution in PDT research due to their unique advantages[32]. One key advantage of nanomaterials is their capability to efficiently load photosensitizers and transport them to cancer cells. Additionally, nanomaterials can be modified to prolong their circulation time in the bloodstream by conferring them with stealth properties and can actively target cancer cells by conjugating with targeting agents such as antibodies and peptides[33]. These functional nanomaterials used in PDT have special optical properties as transducers and are categorized by their diverse properties, such as self-illumination, NIR active nanomaterials, X-ray nano scintillators, or persistent luminescence nanoparticles.
The current approach to light delivery in PDT typically depends on external sources such as lamps, light-emitting diodes (LEDs), and lasers. However, these sources often fall short in adequately delivering the necessary light energy for biological activity to regions deep within the body[30]. Self-illuminated nanomaterials offer a promising solution for targeting deep-seated tumors by combining photosensitizers at the tumor site. Photodynamic therapy (PDT) can be performed without the need for an external light source by using an internal light source that activates the photosensitizers. Various self-illuminating systems, including chemiluminescence, bioluminescence, and Cherenkov radiation, have been investigated for their ability to deliver adequate irradiation for PDT reactions[34]. However, further studies are required to investigate the efficacy of these systems for PDT in the treatment of HCC. Studies of fiber optic-mediated NIR light irradiation using a modified fiber optic tip have demonstrated the light-carrying capacity of blood in swine animal models. This modified tip involved a curved fiber end for targeted light therapy. The NIR light-carrying capacity of blood may allow for fiber-optic mediated light therapy in the liver by allowing light to travel through hepatic vessels to multiple tumor sites[35].
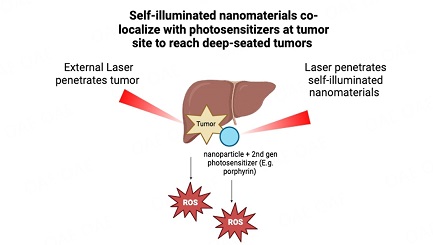
However, further studies are required to investigate the efficacy of these systems for PDT in the treatment of HCC. Advancements in light delivery technologies, especially with the use of fiber optics, have resulted in enhanced clinical approaches for reaching deep tissues during irradiation. However, despite these improvements, issues with the effectiveness of PDT remain, partly due to limitations in the penetration of light. To overcome this, newly developed technologies are being used for the application of nanomaterials. These technologies include self-illuminated nanomaterials, NIR or X-ray activatable nanomaterials, PLNPs as transducers, and the use of implantable light delivery devices. These advancements have demonstrated the effectiveness of PDT both in vitro and in vivo. Noteworthy examples involve the use of functional nanomaterials capable of self-illumination or activation by NIR light and X-rays as transducers, as well as implantable light delivery devices, as illustrated in Figure 1[31].
Figure 1. Self-illuminated nanomaterials co-localize with photosensitizers at tumor sites to reach deep-seated tumors. Created in BioRender.com.
Photosensitizers
Several novel approaches have been adopted to enhance the efficiency of photosensitizers for PDT. These include the attachment of targeting moieties to photosensitizers, employing nanoformulations for their delivery, and integrating PDT with complementary cancer therapies[36]. When developing ideal photosensitizers, key properties to consider are tumor localization, minimal dark toxicity and photosensitivity, high ROS production, and maximum light toxicity.
First-generation photosensitizers clinically approved for PDT cancer treatment
The first-generation photosensitizers approved for clinical use in PDT cancer treatment were Photofrin and Radachlorin[36]. However, they were accompanied by clinical limitations, including skin photosensitivity and limited tissue penetration.
Second-generation photosensitizers
In response to the clinical constraints posed by first-generation photosensitizers, researchers developed second-generation alternatives. These included chlorins, benzoporphyrins, phthalocyanines, and naphthalocyanines, along with their derivatives[36]. Notably, these second-generation photosensitizers offer enhanced characteristics such as deeper tissue penetration, increased production of singlet oxygen, heightened tumor selectivity, expedited tumor uptake (thus reducing the interval between photosensitizer administration and light irradiation), and swifter clearance from normal tissues. Consequently, they minimize photosensitization of normal tissues compared to their first-generation counterparts.
One such approach is to conjugate targeting moieties to photosensitizers[36]. This involves attaching molecules to the photosensitizer that can specifically target cancer cells, increasing the photosensitizer’s concentration at the tumor site and reducing its accumulation in healthy tissues. Another approach is to adopt nanoformulations for photosensitizers, which can enhance their solubility, stability, and bioavailability, thus improving their therapeutic efficacy.
Moreover, the combination of PDT with other cancer therapies is another promising approach[36]. This can include combining PDT with chemotherapy, radiotherapy, or immunotherapy, or using it as an adjuvant therapy to enhance the efficacy of other treatments. When developing ideal photosensitizers, certain properties are necessary for optimal therapeutic efficacy. These properties include tumor localization, minimal dark toxicity, minimal photosensitivity, high ROS production, and maximum light toxicity. Achieving these properties can enhance the specificity and efficacy of PDT while minimizing its side effects.
Photofrin-induced PDT demonstrates prolonged response and good local control for breast carcinoma
Fourteen women, ranging in age from 38 to 67 years old and diagnosed with biopsy-confirmed breast cancer recurrence to the chest wall, received Photofrin-induced PDT at the Leo Jenkins Cancer Center[37]. The treatment was successful in 9 of the 14 patients (64%), as they showed complete necrosis of all detectable or palpable lesions. The treated areas also showed tumor necrosis and re-epithelialization. Some patients also showed initial regression of untreated lesions, indicating that the treatment had a systemic effect. The low-dose Photofrin-induced PDT proved to be an effective treatment for controlling tumor growth, and it also had the added benefit of rapidly reducing the size and drying the surface of the tumor, thereby mitigating wound care concerns. However, wound care was still a challenge for patients with deep tissue diseases (Cuenta, 2004).
Photomed-induced PDT demonstrates effective anticancer properties
A novel photosensitizer, denoted as Photomed, was synthesized and evaluated, particularly focusing on its 3-substituted methyl pyropheophorbide-a derivative[36]. To assess the safety and efficacy of Photomed, researchers conducted a cytotoxicity assay targeting SCC VII cells, a murine squamous cell carcinoma model. This assay was performed through an in vivo anticancer efficacy study using mice bearing SCC VII tumors.
The objective of the study was to compare changes in tumor surface characteristics in mice bearing SCC VII tumors pre- and post-initial PDT utilizing Photofrin, Radachlorin, Photomed, and PBS[36]. Various analyses were conducted to monitor the outcomes, encompassing tumor size measurements, surface examinations, and histological assessments. The results unveiled that Photomed-induced PDT demonstrated the most effective anticancer properties compared to other treatments. It led to complete tumor cell eradication, an augmented necrotic portion, and a diminished mitotic area, signifying substantial antitumor efficacy.
Low O2 generation and photobleaching
The drawback of low O2 generation stems from the decomposition of ICG molecules (photobleaching) which reduces the effectiveness of phototherapy[38]. Suggested strategies involve embedding ICG into engineered nanoparticles, encompassing options such as macromolecular nanoparticles, 2D nanomaterials, liposomes, and noble metal nanostructures. This integration led to the improved photostability of ICG and enhanced intramolecular interactions, resulting in improved PTT through ICG aggregation within the nanoparticles. However, a major challenge with ICG-loaded nanoparticles is the aggregation of ICG molecules in the nanoparticles, which causes an electronically excited state quenching effect. This effect limits the PDT efficacy due to aggregation-caused quenching (ACQ).
The Pt-ICG/PES platform was developed to overcome this, as it offers a promising way to enhance the immune response against tumors and the photophysical properties of ICG, as shown in Figure 2[38]. This involves the intercalation of temperature-sensitive spacers (PES) into a complex of cisplatin and ICG. By adjusting the PES/ICG ratio, the anti-tumor immune response is enhanced. Furthermore, the nanoparticles, which encompass diverse metal ions (platinum, magnesium, and manganese) and spacers (PES and PEA), exhibited substantial enhancements in quantum yield (∆Φ). This improvement stemmed from their ability to suppress aggregation-caused quenching (ACQ) and mitigate the photobleaching of ICG.
Figure 2. Combination of PDT with other cancer therapies, such as ICG/PES increases efficiency, allows for multiple benefits, and overcomes drawbacks of low O2 generation and photobleaching. Created in BioRender.com.
The results from this study showed that Pt-ICG/PES exhibited a sevenfold increase in Φ∆ compared to the free ICG, attributed to the inhibition of ICG aggregation, which enhances the O2 generation by the photosensitizer[38]. The Pt-ICG/PES platform activates the ICD effect by increasing DAMP levels (CRT, HMGB-1, and ATP) upon NIR irradiation, which evokes a systemic immune response against the tumor. In addition, Pt-ICG/PES also profits from the M1-phenotype macrophage polarization, which has been shown to be effective in stimulating antitumor immunity. Furthermore, the platform can alleviate the hypoxic microenvironment and facilitate tumor-targeted delivery through mild hyperthermia-induced PTT, which triggers a temperature-sensitive hydrophilic-hydrophobic transition.
While this study presents a promising strategy with the potential for clinical translation to enhance the efficacy of PDT and integrate three modalities (PDT, PTT, and chemotherapy) into a single platform, there remains considerable scope for improvement. By incorporating additional immunotherapies, such as immune checkpoint blockade immunotherapy, with Pt-ICG/PES, distant tumors could be eliminated more efficiently. Overall, this approach demonstrates a robust systemic antitumor immune response and holds great potential for future development.
In vitro ICG-NIR study demonstrates increased ROS production in HCC
In an in vitro study analyzing HT-1080 cells transduced to express OATP183, cells were treated with ICG for 4 h[39]. Four groups of cells, control without ICG or laser therapy, ICG treated with laser therapy, laser therapy without ICG treatment, and ICG treatment without laser therapy, were irradiated with a laser of wavelength 808 nm and a power output of 15 W. This allowed for an independent assessment of each variable as well as an analysis of the combined effect of ICG treatment and PDT. Cells were irradiated twice, once after four hours of ICG treatment and again 24 h after the initial irradiation. All groups were analyzed for reactive oxygen species (ROS).
In this study, it was observed that cells treated with ICG-NIR had a significantly higher level of ROS production in comparison to the cells treated with ICG alone[39]. Additionally, the assessment of cell viability showed that the ICG-NIR-treated cells had fewer viable cells compared to the ICG-only treated group. These results imply that the combined treatment of ICG and NIR has a more pronounced effect on the production of ROS and cell viability than ICG treatment alone.
Advancement in nanotechnology
PDT-PTT offers distinctive advantages over alternative therapies, characterized by minimal cytotoxicity, non-invasive nature, ability for remote control, and low occurrence of side effects[40]. Operating within the NIR phototherapeutic range for cancer treatment (700 to 1,100 nm), laser energy absorption by tissues and blood is minimal, allowing for effective cancer treatment through thermal ablation. NIR-stimulated PSs generate ROS, including free radicals, peroxides, and singlet oxygen, capable of eradicating tumor cells in PDT. The integration of diverse nanostructures with photosensitizers yields a dual PDT-PTT within a single nanoplatform, thereby enhancing therapeutic efficacy against cancer.
Combination of optical excitation and ultrasonic detection for deep-seated tumors
The second NIR-II optical window, spanning from 1,000 to 1,350 nm, permits the excitation laser to penetrate deeper into tissues. However, there is a scarcity of NIR-II agents available for diagnosing and conducting photothermal therapy for small HCC (SHCC). Remarkably, there has been minimal focus on orthotopic tumor model-based research, likely due to challenges related to tissue penetration or the biocompatibility of contrast agents. Introducing a novel targeted plasmonic-doped melanin-based theranostic nanoagent (Pt@PDA-c) addresses these limitations, offering NIR-II photoacoustic (PACT) guidance for early detection of primary SHCC and effective non-invasive photothermal ablation of orthotopic tumors. Efficient in-vitro targeted tumor-binding, reliable biosafety, and NIR-II laser irradiation resulted in minimal harm to healthy liver tissue.
Nanomaterials have the potential to be utilized in encapsulating ICG, which can offer several benefits shown in Figure 3[40,41]. Firstly, nanomaterials can shield ICG from decomposition and clearance by leveraging the enhanced EPR effect. Secondly, they can improve the chemical and photothermal stability of ICG, leading to better performance. Finally, by designing nanomaterials to precisely target the desired site, their efficacy can be further amplified.
Figure 3. ICG combined with nanomaterials protects the ICG from decomposition and elimination through the enhanced EPR effect, and improves the chemical and photothermal stability of ICG. Created in BioRender.com.
Gold nanostructures
Gold nanoparticles coated with gadolinium ions were designed, forming an ultra-small size core-shell nanosystem[42]. These gold nanostructures were then engineered to incorporate matrix metalloproteinase-2 (MMP-2) and IR820, facilitating synergistic therapies (PTT-PDT) against liver cancer. The nanosystem underwent in vivo metabolism through biodegradation, facilitated by its acid-stimulated degradation nature, enabling bimodal imaging. The presence of MMP-2 enabled the nanomaterials to exhibit exceptional specific targeting capability. Through in vivo investigations, it was demonstrated that the nanomaterials achieved enhanced synergistic PDT-PTT efficacy upon laser treatment, effectively suppressing tumor growth. This study concludes that the gold nanostructure-based nanosystem exhibits high efficiency against liver cancer, presenting a dual imaging capability.
Gold nanomaterials are extensively employed in biomedical research due to their distinctive physicochemical properties and excellent biocompatibility. They serve various purposes, including acting as drug delivery systems, imaging agents, and therapeutic materials. In this study, ultra-small gold nanoparticles (AuNPs) were synthesized and combined with gadolinium ions to create a spherical self-assembly structure. Following this, the nanoparticles were conjugated with matrix metalloproteinase-2 (MMP-2) and loaded with the photosensitive drug IR820 for a combined photothermal and photodynamic therapy approach targeting liver cancer. The resulting nanoprobes underwent in vivo degradation under real-time dual-mode imaging due to their acid-responsive degradation characteristics. Additionally, the nanoprobes exhibited excellent tumor-targeting ability through surface modification with MMP-2. Treatment experiments in vivo revealed augmented effectiveness of the photodynamic and photothermal combination therapy facilitated by the nanoprobes upon laser irradiation, leading to significant inhibition of tumor growth. Therefore, the nanoprobes hold great potential for antitumor therapy guided by real-time dual-mode imaging of liver cancer.
Nanocomplexes for combined phototherapies for deep-seated tumors
The use of synergistic phototherapies has been found to increase the effectiveness of cancer treatments against various types of cancers[43]. However, the challenge lies in creating a delivery approach that can target specific and deep-penetrating tumors. Chuang et al. have pioneered a groundbreaking approach by harnessing stem cells for the precise delivery of photodynamic and photothermal agents in cancer therapy[43]. In this innovative method, gold nanorods (AuNRs) serve as photothermal agents, complemented by Chlorin e6 (Ce6) for photodynamic therapy (PDT). Stem cells, specifically adipose-derived stem cells (ADSC), were further loaded with PEG, PEI, and Ce6-functionalized AuNRs. Upon NIR light irradiation at 808 nm, the photothermal treatment is activated, facilitating the controlled release of Ce6 from the stem cells into the tumor microenvironment.
The study has shown that ADSCs can serve as a promising vehicle for co-delivery of AuNRs and Ce6 for cancer treatment[43]. APP/Ce6 was used to construct APP/Ce6-loaded ADSCs, and exhibited several advantageous features, including robust cell viability, effective tumor migration and infiltration, and potent photothermal and photodynamic effects. The tumor-homing ability and anticancer efficacy of these loaded ADSCs were confirmed through in vitro experiments. In a murine model of colon cancer, significant tumor suppression was observed in mice treated with APP/Ce6-loaded ADSCs followed by light irradiation, as shown in Figure 4. These findings underscore the potential of stem cell-based combined photothermal therapy (PTT) and photodynamic therapy (PDT) as a promising strategy for cancer treatment.
Figure 4. Novel gold nanorod (AuNR)/Ce6 is loaded into stem cells as a live carrier to codeliver and act as photothermal and photodynamic agents. Created in BioRender.com.
NIR-II combined with novel nanoagent Pt@PDA-c for deep-seated tumors
The utilization of the second near-infrared window (NIR-II, 1,000-1,350 nm) is advantageous in medical settings as it allows deeper tissue penetration for excitation lasers. However, the availability of NIR-II agents for diagnosing and treating small hepatocellular carcinoma (SHCC) with PTT is limited. To address this gap, a novel targeted plasmonic-doped, melanin-based theranostic nanoagent (Pt@PDA-c) was developed. This innovative agent, combined with NIR-II photoacoustic (PACT) guidance and phototheranostics, was found to aid early detection of small primary HCC and enable the efficient non-invasive photothermal ablation of orthotopic tumors. Efficient in-vitro targeted tumor-binding, reliable biosafety, and NIR-II laser irradiation resulted in minimal damage to healthy liver tissue[44].
Design of AIE-PSs for efficient ROS generation
To improve ROS generation, it is essential to design aggregation-induced emission photosensitizers (AIE-PSs) that enhance efficiency[45]. The AIE phenomenon relies on restricted intermolecular motion (RIM), which prevents intersystem crossing (ISC) and leads to excited molecules transitioning to the T1 state through radiative transition when in the aggregated state. PSs in the T1 state can react with surrounding substrates, promoting the production of various ROS. AIE-PSs stimulate radiative pathways while inhibiting non-radiative decay pathways during aggregation, resulting in increased production of ROS. This property makes them ideal for PDT and could make them the dominant molecule in this field.
Development of high-efficiency AIE-PSs improves ROS generation for liver cancer
PDT using ICG produces reactive oxygen species (ROS) upon exposure to light of a specific wavelength[46]. This ROS production is crucial for efficacy. It is worth noting that photosensitizers are not only responsible for ROS generation but also possess fluorescence properties, which can be exploited to combine optical imaging with PDT. By using fluorescence, it is possible to visualize the distribution of photosensitizers in the targeted tissues and monitor the treatment process in real time. Therefore, photosensitizers are a versatile and essential component of PDT, offering both therapeutic and diagnostic benefits.
AIE quantum dots with effective PDT demonstrate liver cell tracking for HCC
A new nanoprobe that is active with AIE was presented as a cell tracker suitable for prolonged fluorescent imaging both in vitro and in vivo[47]. AIE-active nanoprobes, called TNZ2tPPI-Tat NPs, tracked hepatoma cell MHCC97-H for over 5 days through real-time fluorescence feedback. Three kinds of liver cells (Lo2, MHCC97-H, and Hep 3B) were successfully labeled and tracked by TNZ2tPPI-Tat NPs. In vivo results showed that TNZ2tPPI-Tat NPs ensured fluorescence for more than 26 days, indicating significant biocompatibility, biosafety, stability, and increased duration of circulation. In vitro, normal Lo2 cells were incubated with three probes with varying concentrations (ranging from 0 to 400 μg/mL). After 24 h, cell viability was assessed using the CCK8 method. Even at a concentration of 400 μg/mL, the commercial probes of Cy5 and AF647 had higher cell viability. Similarly, at a concentration of 400 μg/mL, 90% of cell viability remains when incubated with TNZ2tPPI-Tat dots, indicating the similarity of biosafety of AIE dots with commercial probes. Overall, TNZ2tPPI-Tat nanoparticles demonstrated better performance compared to commercial probes Cy5 and AF647 in terms of anti-photobleaching, long-term fluorescence tracking, and biocompatibility both in vivo and in vitro.
Effective AIE NIR PDT demonstrates increased ROS production, long-term tracking, and photodynamic ablation
A highly efficient AIE-active NIR emissive photosensitizer was synthesized, incorporating rhodanine as an electron acceptor and triphenylvinylthiophene as an electron donor. Subsequently, this photosensitizer was encapsulated within Pluronic F127 to develop NIR organic fluorescent nanoparticles. NIR fluorescent bioimaging and PDT ability of TPVTR dots were evaluated in vitro and in vivo[48].
In vitro, the evaluation of TPVTR dots efficacy in PDT was assessed using CCK-8 assays, targeting HepG2 cells as an in vitro model for HCC. At a concentration of 50 μg·mL-1, TPVR dots exhibited minimal cytotoxicity in the absence of light and demonstrated excellent compatibility with living cells, as evidenced by a cell viability rate of nearly 80%. However, upon exposure to light (100 mW·cm-2, 10 min), the viability of HepG2 cells drops significantly to less than 50% after treatment with 25 μg·mL-1 of TPVTR dots, demonstrating the effectiveness of PDT[48].
In vivo, tumor ablation was measured to evaluate the efficacy of PDT, as shown in Figure 5. Nude mice were subjected to subcutaneous implantation of HepG2 cells at a concentration of 5 × 106 cells per mouse, followed by random division into four groups. One group received saline injection, another underwent light irradiation (400-750 nm, 100 mW·cm-2), while the third group was treated with intratumoral injection of TPVTR dots. The fourth group received light irradiation (400-750 nm, 100 mW·cm-2) after intratumoral injection of TPVTR dots. Treatment was initiated when tumors reached 100-200 mm3. Mice were monitored every two days for up to 22 days for body weight and tumor size to determine PDT efficiency in vivo. To mitigate skin damage, the tumor site was not exposed to irradiation for one minute following each irradiation cycle. Additionally, the temperature of the tumor site was closely monitored, ensuring it remained below 42 °C both before and after light irradiation (in the range of 400-750 nm) for a duration of 10 min, with a minimal temperature variation of only 1.67 °C. The tumor in the saline group grew rapidly and did not differ significantly from the control groups. On the other hand, PDT significantly ablated tumor growth after 21 days, validating its effectiveness. Additionally, no notable differences in body weight were observed among the various groups. TPVTR dots demonstrated their efficient PDT efficacy in vivo through a substantial reduction in tumor size.
Figure 5. Photodynamic therapy with nanoprobe (AuNPs and MMP-2) demonstrates increased efficiency and additional benefits in comparison to the standard control of PBS and laser treatment. Created in BioRender.com.
Limitations of AIE PSs
Despite the promising potential of AIE PSs in tumor PDT, several limitations remain in their design that must be addressed[46]. Additionally, PDT approaches utilizing AIE PSs remain in the early stages and require continuous research efforts to be integrated into clinical practice. The primary challenges faced by AIE PSs in tumor PDT include shallow tissue penetration, limited light penetration depth, and the necessity of combining other therapies to effectively treat tumors. To enhance the overall efficacy of PDT in tumor treatment, the design and implementation of efficient AIE PSs must be further investigated, as well as the development of comprehensive PDT strategies to address these challenges.
Combination therapy with other modalities to increase the efficacy of PDT
Novel combined treatments: metronomic capecitabine
A promising avenue of research in cancer treatment is the development of novel combined treatments. Historically, first-line therapy with Sorafenib has proven to be ineffective, resulting in tumor progression and liver toxicity. Metronomic capecitabine (MC) was introduced as a safe, effective, and well-tolerated systemic treatment upgrade in HCC patients, demonstrating lower tumor burden, lower prevalence of portal vein thrombosis, and better cancer stages[49]. The effectiveness of metronomic capecitabine can be attributed to its ability to significantly increase the radiosensitivity of A549 cells. It achieves this via the inhibition of proliferation, leading to a significant rise in cell death and halting the progression of cells into the G0/G1 phase following radiotherapy with Y-[60Co][50].
The usage of capecitabine has been combined with photosensitizers, such as indocyanine green (ICG) and methylene blue (MB), and incorporated into nanoparticles to create a dual-targeting drug delivery system in prostate cancer. The photothermal conversion-triggered release of modified nanoparticles significantly induced apoptosis in both DU145 and P3 cells, with an apoptotic rate of approximately 80% on DU145 cancer cells specifically[51]. However, it should be noted that there have been limited studies of this novel combination treatment on liver cells and HCC, which indicates a need for further research in this area.
LEM and hydrogen gas inhalation therapy
In the clinical setting, patients received PDT with ICG in conjunction with Lentinula edodes mycelia (LEM) and hydrogen gas inhalation therapy. LEM is intended to exhibit CTLA-4 inhibitor effects similar to the common antibody drug ipilimumab. Its mechanism involves delaying tumor progression by reinstating antitumor immunity and increasing regulatory T cells (Tregs) in cancer-afflicted mice. Furthermore, unlike conventional antibody drugs, LEM contains various constituents imbued with antioxidant and antitumor properties. Hydrogen gas inhalation therapy has been shown to reduce inflammation in the body by removing ROS. Additionally, it diminished programmed cell death protein 1 (PD-1) expression in cytotoxic T-lymphocytes (CTLs), thereby augmenting the ratio of negative CTLs to positive ones[52].
ICG liposomes with LEM and hydrogen gas
Recent studies have found drugs that allow ICG to convert into liposomes or micelles, which exhibit accumulation in cancer cells via the enhanced permeability and retention (EPR) effect[53]. Leveraging this phenomenon enables the selective targeting of cancer cells when combined with PDT. Utilizing ICG liposomes alongside LEM and hydrogen gas augments the body’s innate immunity against tumors, which allows cancer cells to be eradicated without burdening patients with side effects[52].
ICG liposomes were synthesized by combining 5 mg of ICG with 25 mL of liposomes[52]. The ICG liposomes were administered to a patient with stage II esophagus carcinoma. The patient elected to undergo the alternate treatment method of PDT instead of the recommended surgery. ICG has been demonstrated to function in the esophagus similar to the liver. Following ICG administration, PDT was performed using an endoscopic laser emitting light with a wavelength of 810 nm. The patient underwent a total of six PDT sessions, each with a duration of 20 min. 13 months after the initial PDT treatment, the patient showed no tumor presence. Throughout the PDT regimen, the patient concurrently received LEM and hydrogen gas inhalation therapy, with no observed adverse effects.
In a separate case involving a patient with hypopharyngeal cancer, both chemotherapy and radiation therapy proved ineffective[52]. Subsequently, the patient underwent intravenous administration of 50 mL of ICG liposomes, followed by PDT the following day utilizing an 810 nm wavelength laser. The PDT was performed using a fiber optic and lasted 40 min. 1 month following initial treatment, tumor shrinkage was observed, as well as improvement of lymphadenopathy. Similar to the previous patient, PDT was administered concurrently with LEM and hydrogen gas inhalation therapy, without any reported adverse effects.
The synergistic approach of combining PDT with LEM and hydrogen gas inhalation therapy exhibits encouraging outcomes in bolstering the body’s innate immunity against tumors while eradicating cancerous cells. The use of ICG liposomes further allows for selective targeting of cancer cells, minimizing side effects. The successful case study of a patient with stage II esophagus carcinoma demonstrates the potential of this treatment approach. Further research and clinical trials are needed to validate and optimize this combination therapy for broader application in cancer treatment.
ICG liposomes with NIR-II fluorescence imaging via computational modeling
In comparison with conventional optical imaging methodologies, NIR-II fluorescence imaging combined with the clinical administration of ICG is underscored by its ability to provide detailed structural and functional images in living organisms with remarkable clarity and contrast, which has been effectively applied to navigate human liver cancer surgery under bright-field conditions. However, improving the intensity of ICG’s NIR-II emission in vivo remains a significant challenge, with factors such as the excitation absorption and coefficient and fluorescence quantum yield (QY) remaining, despite advancements in molecular engineering strategies and advanced technology aimed at enhancing the imaging performance of cyanine dyes and expanding their phototheranostic applications[51].
A novel computational method regarding excitation-dependent dual color NIR-II imaging employing two distinct S-Lipo-cyanine dyes was proposed as a revolutionary way to monitor complex biological processes. The encapsulation of ICG in liposomes was corroborated by computational modeling and was found to not only improve NIR-II fluorescence imaging, but also preserve the metabolic pathway, which holds great promise for future clinical translation[51]. Additionally, the upscaling of S-Lipo-ICG facilitates tumor surgery guided by imaging in rabbit models, demonstrating the practical application of this research. In this context, computational biology has the potential to enhance imaging agents’ efficiency, offering a new approach to understanding agent-biological system interactions at the molecular level. This could lead to more effective therapies, revolutionizing medical imaging and treatment.
DISCUSSION
ICG is a fluorescent dye that has been used in the clinical setting for decades as a method of imaging primary hepatocellular carcinoma using NIR[54]. It has a long record of clinical safety and efficacy, which is a significant benefit for its potential use as a photosensitizer for PDT. Current treatment options for hepatocellular carcinoma are slim and often ineffective in more severe HCC cases[55-58]. Therefore, PDT offers a safer and more effective therapeutic option to treat HCC for patients who are unable to receive surgical resection or transplantation.
ICG PDT has been shown to effectively eliminate liver cancer cells in vitro and in vivo, and has demonstrated clinical efficacy in many forms of cancer in the clinical setting[12,13,21,59-62]. ICG therapy in the clinical setting has been performed through the use of ICG liposomes rather than direct administration of ICG intravenously. Further research is required in order to investigate the efficacy of direct administration of ICG for cases of HCC.
Current struggles in the optimization of PDT in the clinical setting revolve around three central issues: limited light delivery, optimizing photosensitizers, and low O2 generation. Light delivery mechanisms require unique engineering solutions. Promising developments in light delivery mechanisms include the inclusion of ICG in nanostructures. Current photosensitizers pose the issue of differing evacuation rates in the liver, which leads to variable time between photosensitizer administration and PDT treatment. O2 generation is an essential mechanism of PDT, as it is necessary to result in tumor cell apoptosis. Increasing O2 generation through photosensitizers and innovative light delivery solutions can help create safe and effective PDT options for the clinical setting.
ICG has the advantage of proven clinical safety through its extensive historical use in the clinic. Combining ICG PDT with LEM and hydrogen gas inhalation therapy offers a simple solution to the limited O2 production that is commonly seen with ICG PDT. Additionally, the use of AIE PSs offers insight into the improved efficacy of engineered photosensitizers and their ability to demonstrate efficacy in the clinical setting.
For patients with HCC who are ineligible for resection or transplant, the use of PDT can offer a safe and reliable therapeutic option that was previously unavailable. ICG PDT offers the first step of this process, as approval for use in the clinical setting could be significantly streamlined due to ICG already being an approved and widely used photosensitizer in the clinical setting. To further improve the efficacy of PDT, developments in photosensitizer technology such as the use of AIE-PSs, as well as combination therapies including LEM or hydrogen gas inhalation, should be considered for widespread clinical use as well. Ultimately, further research is required before such advancements can be implemented.
CONCLUSION
The field of photodynamic therapy (PDT) presents a promising avenue for cancer treatment. Nonetheless, there remain limitations that require attention to maximize its efficacy. Improved light delivery technologies, such as the use of nanomaterials and self-illuminating systems, can help overcome the challenge of light penetration and enhance the effectiveness of PDT. Additionally, the development of novel photosensitizers and their conjugation with targeting moieties can improve tumor selectivity and minimize side effects. Furthermore, advancements in nanotechnology, including the use of nanomaterials for encapsulating photosensitizers, offer potential benefits in terms of stability, targeting, and therapeutic efficacy. The integration of PDT with other cancer therapies and the design of aggregation-induced emission photosensitizers (AIE-PSs) can further enhance ROS generation and improve the overall effectiveness of PDT. The novelty of this work necessitates continuous research and innovation in these areas for the advancement of PDT as a powerful, non-invasive and effective treatment option for cancer.
DECLARATIONS
Authors’ contributions
Contributions to conception, investigation, and interpretations: Jo E, Savsani K, Lee SD
Writing of the manuscript draft and revisions: Jo E, Savsani K, Alfonso A, Park A
Project administration by providing comments and feedback: Sarkar D, Kinsey N, Sombommatsu Y,
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2024.
REFERENCES
1. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6.
2. Asafo-Agyei KO, Samant H. Hepatocellular carcinoma. Available from: http://www.ncbi.nlm.nih.gov/books/NBK559177/ [Last accessed on 8 Apr 2024].
3. Balogh J, Victor D 3rd, Asham EH, et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma 2016;3:41-53.
4. Colagrande S, Regini F, Taliani GG, Nardi C, Inghilesi AL. Advanced hepatocellular carcinoma and sorafenib: diagnosis, indications, clinical and radiological follow-up. World J Hepatol 2015;7:1041-53.
5. Allaire M, Goumard C, Lim C, Le Cleach A, Wagner M, Scatton O. New frontiers in liver resection for hepatocellular carcinoma. JHEP Rep 2020;2:100134.
7. Madkhali AA, Fadel ZT, Aljiffry MM, Hassanain MM. Surgical treatment for hepatocellular carcinoma. Saudi J Gastroenterol 2015;21:11-7.
8. Olthoff KM. Surgical options for hepatocellular carcinoma: resection and transplantation. Liver Transpl Surg 1998;4:S98-104.
9. Chapman WC, Klintmalm G, Hemming A, et al. Surgical treatment of hepatocellular carcinoma in North America: can hepatic resection still be justified? J Am Coll Surg 2015;220:628-37.
10. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet 2022;400:1345-62.
11. Pravisani R, Baccarani U, Isola M, et al. Impact of surgical complications on the risk of hepatocellular carcinoma recurrence after hepatic resection. Updates Surg 2018;70:57-66.
12. Kaneko J, Kokudo T, Inagaki Y, Hasegawa K. Innovative treatment for hepatocellular carcinoma (HCC). Transl Gastroenterol Hepatol 2018;3:78.
13. Shirata C, Kaneko J, Inagaki Y, et al. Near-infrared photothermal/photodynamic therapy with indocyanine green induces apoptosis of hepatocellular carcinoma cells through oxidative stress. Sci Rep 2017;7:13958.
14. Yao S, Zhang L, Ma J, Jia W, Chen H. Precise right hemihepatectomy for the treatment of hepatocellular carcinoma guided by fusion ICG fluorescence imaging. J Cancer 2020;11:2465-75.
15. Sebagh M, Desterke C, Feray C, et al. Indocyanine green fluorescence patterns of hepatocellular carcinoma correlate with pathological and molecular features. HPB 2023;25:198-209.
16. Huang SW, Ou JJ, Wong HP. The use of indocyanine green imaging technique in patient with hepatocellular carcinoma. Transl Gastroenterol Hepatol 2018;3:95.
17. Liu F, Wang H, Ma W, et al. Short- and long-term outcomes of indocyanine green fluorescence navigation- versus conventional-laparoscopic hepatectomy for hepatocellular carcinoma: a propensity score-matched, retrospective, cohort study. Ann Surg Oncol 2023;30:1991-2002.
18. Urade T, Sawa H, Iwatani Y, et al. Laparoscopic anatomical liver resection using indocyanine green fluorescence imaging. Asian J Surg 2020;43:362-8.
19. Qian X, Hu W, Gao L, et al. Trans-arterial positive ICG staining-guided laparoscopic liver watershed resection for hepatocellular carcinoma. Front Oncol 2022;12:966626.
20. Nanashima A, Hiyoshi M, Imamura N, Yano K, Hamada T, Kai K. Recent advances in photodynamic imaging and therapy in hepatobiliary malignancies: clinical and experimental aspects. Curr Oncol 2021;28:4067-79.
21. Park JS, Park S, Park SJ, Kim SK. Synergistic effects of concurrent photodynamic therapy with indocyanine green and chemotherapy in hepatocellular carcinoma cell lines and mouse models. J Photochem Photobiol B 2023;239:112642.
22. Wang Q, Zhong YJ, Yuan JP, et al. Targeting therapy of hepatocellular carcinoma with doxorubicin prodrug PDOX increases anti-metastatic effect and reduces toxicity: a preclinical study. J Transl Med 2013;11:192.
23. Zhang Z, Hu K, Miyake K, et al. A novel patient-derived orthotopic xenograft (PDOX) mouse model of highly-aggressive liver metastasis for identification of candidate effective drug-combinations. Sci Rep 2020;10:20105.
24. Hong F, Park JS, Kim SW, Park SJ, Kim SK. Near-infrared phototherapy for patient-derived orthotopic xenograft model of hepatocellular carcinoma in combination with indocyanine green. J Photochem Photobiol B 2020;209:111938.
25. Hu B, Li H, Guo W, et al. Establishment of a hepatocellular carcinoma patient-derived xenograft platform and its application in biomarker identification. Int J Cancer 2020;146:1606-17.
26. Zhang Y, Zhang GL, Sun X, et al. Establishment of a murine breast tumor model by subcutaneous or orthotopic implantation. Oncol Lett 2018;15:6233-40.
27. Kumar A, Moralès O, Mordon S, Delhem N, Boleslawski E. Could photodynamic therapy be a promising therapeutic modality in hepatocellular carcinoma patients? A critical review of experimental and clinical studies. Cancers 2021;13:5176.
28. Peng Z, Lv X, Huang S. Photoimmunotherapy: a new paradigm in solid tumor immunotherapy. Cancer Control 2022;29:107327482210888.
29. Sevieri M, Silva F, Bonizzi A, et al. Indocyanine green nanoparticles: are they compelling for cancer treatment? Front Chem 2020;8:535.
30. Gunaydin G, Gedik ME, Ayan S. Photodynamic therapy-current limitations and novel approaches. Front Chem 2021;9:691697.
31. Sun B, Bte Rahmat JN, Zhang Y. Advanced techniques for performing photodynamic therapy in deep-seated tissues. Biomaterials 2022;291:121875.
32. Dinakaran D, Wilson BC. The use of nanomaterials in advancing photodynamic therapy (PDT) for deep-seated tumors and synergy with radiotherapy. Front Bioeng Biotechnol 2023;11:1250804.
33. Bae KH, Chung HJ, Park TG. Nanomaterials for cancer therapy and imaging. Mol Cells 2011;31:295-302.
34. Kamkaew A, Cheng L, Goel S, et al. Cerenkov radiation induced photodynamic therapy using chlorin e6-loaded hollow mesoporous silica nanoparticles. ACS Appl Mater Interfaces 2016;8:26630-7.
35. Du W, Wang Y, Luo Q, Liu BF. Optical molecular imaging for systems biology: from molecule to organism. Anal Bioanal Chem 2006;386:444-57.
36. Kim J, Kim J, Yoon H, Chae YJ, Rhew K, Chang JE. The in vitro and in vivo anticancer effect of photomed for photodynamic therapy: comparison with photofrin and radachlorin. Curr Issues Mol Biol 2023;45:2474-90.
37. Cuenca RE, Allison RR, Sibata C, Downie GH. Breast cancer with chest wall progression: treatment with photodynamic therapy. Ann Surg Oncol 2004;11:322-7.
38. Zhao H, Xu J, Feng C, et al. Tailoring aggregation extent of photosensitizers to boost phototherapy potency for eliciting systemic antitumor immunity. Adv Mater 2022;34:e2106390.
39. Tseng HC, Kuo CY, Liao WT, Chou TS, Hsiao JK. Indocyanine green as a near-infrared theranostic agent for ferroptosis and apoptosis-based, photothermal, and photodynamic cancer therapy. Front Mol Biosci 2022;9:1045885.
40. Sekar R, Basavegowda N, Thathapudi JJ, et al. Recent progress of gold-based nanostructures towards future emblem of photo-triggered cancer theranostics: a special focus on combinatorial phototherapies. Pharmaceutics 2023;15:433.
41. Mahmut Z, Zhang C, Ruan F, et al. Medical applications and advancement of near infrared photosensitive indocyanine green molecules. Molecules 2023;28:6085.
42. Li B, Sun L, Li T, et al. Ultra-small gold nanoparticles self-assembled by gadolinium ions for enhanced photothermal/photodynamic liver cancer therapy. J Mater Chem B 2021;9:1138-50.
43. Chuang CC, Chen YN, Wang YY, et al. Stem cell-based delivery of gold/chlorin e6 nanocomplexes for combined photothermal and photodynamic therapy. ACS Appl Mater Interfaces 2020;12:30021-30.
44. Qi S, Zhang Y, Liu G, et al. Plasmonic-doped melanin-mimic for CXCR4-targeted NIR-II photoacoustic computed tomography-guided photothermal ablation of orthotopic hepatocellular carcinoma. Acta Biomater 2021;129:245-57.
45. Ni J, Wang Y, Zhang H, Sun JZ, Tang BZ. Aggregation-induced generation of reactive oxygen species: mechanism and photosensitizer construction. Molecules 2021;26:268.
46. Meng Z, Xue H, Wang T, et al. Aggregation-induced emission photosensitizer-based photodynamic therapy in cancer: from chemical to clinical. J Nanobiotechnology 2022;20:344.
47. Teng M, Chen Y, Xie Y, et al. Bright near-infrared aggregation-induced emission dots for long-term bioimaging in vitro/vivo. Dyes Pigments 2021;195:109679.
48. Xia Q, Chen Z, Zhou Y, Liu R. Near-infrared organic fluorescent nanoparticles for long-term monitoring and photodynamic therapy of cancer. Nanotheranostics 2019;3:156-65.
49. Trevisani F, Brandi G, Garuti F, et al. Metronomic capecitabine as second-line treatment for hepatocellular carcinoma after sorafenib discontinuation. J Cancer Res Clin Oncol 2018;144:403-14.
50. Granito A, Marinelli S, Terzi E, et al. Metronomic capecitabine as second-line treatment in hepatocellular carcinoma after sorafenib failure. Dig Liver Dis 2015;47:518-22.
51. Ma Z, Ji T, Ji G, Niu Q, Han W. Facile construction of dual-drug loaded nanoparticles for improvement synergistic chemotherapy in prostate cancer. Int J Polym Mater Polym Biomater 2023;72:856-66.
52. Yorozu K, Kaibori M, Kimura S, et al. Experience with photodynamic therapy using indocyanine green liposomes for refractory cancer. J Pers Med 2022;12:1039.
53. Gu Z, Da Silva CG, Van der Maaden K, Ossendorp F, Cruz LJ. Liposome-based drug delivery systems in cancer immunotherapy. Pharmaceutics 2020;12:1054.
54. Inagaki FF, Takemura N, Ito K, Mihara F, Kurokawa T, Kokudo N. Intraoperative indocyanine green fluorescence navigation facilitates complete removal of lymph node metastases from hepatocellular carcinoma. Glob Health Med 2021;3:406-8.
55. Chow AK, Yau SW, Ng L. Novel molecular targets in hepatocellular carcinoma. World J Clin Oncol 2020;11:589-605.
56. Hoshida Y, Fuchs BC, Tanabe KK. Prevention of hepatocellular carcinoma: potential targets, experimental models, and clinical challenges. Curr Cancer Drug Targets 2012;12:1129-59.
57. Chen Z, Xie H, Hu M, et al. Recent progress in treatment of hepatocellular carcinoma. Am J Cancer Res 2020;10:2993-3036.
58. Chakraborty E, Sarkar D. Emerging therapies for hepatocellular carcinoma (HCC). Cancers 2022;14:2798.
59. Tsuda T, Kaibori M, Hishikawa H, et al. Near-infrared fluorescence imaging and photodynamic therapy with indocyanine green lactosome has antineoplastic effects for hepatocellular carcinoma. PLoS One 2017;12:e0183527.
60. Qi S, Liu G, Chen J, et al. Targeted multifunctional nanoplatform for imaging-guided precision diagnosis and photothermal/photodynamic therapy of orthotopic hepatocellular carcinoma. Int J Nanomed 2022;17:3777-92.
61. Shemesh CS, Hardy CW, Yu DS, Fernandez B, Zhang H. Indocyanine green loaded liposome nanocarriers for photodynamic therapy using human triple negative breast cancer cells. Photodiagnosis Photodyn Ther 2014;11:193-203.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Jo E, Savsani K, Alfonso A, Park A, Lee SD, Sakar D, Kinsey N, Sambommatsu Y, Imaid D, Khan A, Sharma A, Saeed M, Kumaran V, Cotterell A, Levy M, Bruno D. Photodynamic effect of indocyanine green and its application to hepatocellular carcinoma treatment. J Cancer Metastasis Treat 2024;10:16. http://dx.doi.org/10.20517/2394-4722.2024.18
AMA Style
Jo E, Savsani K, Alfonso A, Park A, Lee SD, Sakar D, Kinsey N, Sambommatsu Y, Imaid D, Khan A, Sharma A, Saeed M, Kumaran V, Cotterell A, Levy M, Bruno D. Photodynamic effect of indocyanine green and its application to hepatocellular carcinoma treatment. Journal of Cancer Metastasis and Treatment. 2024; 10: 16. http://dx.doi.org/10.20517/2394-4722.2024.18
Chicago/Turabian Style
Jo, Ester, Kush Savsani, Anjelica Alfonso, Andrew Park, Seung Duk Lee, Devanand Sakar, Nathaniel Kinsey, Yuzuru Sambommatsu, Daisuke Imaid, Aamir Khan, Amit Sharma, Muhammad Saeed, Vinay Kumaran, Adrian Cotterell, Marlon Levy, David Bruno. 2024. "Photodynamic effect of indocyanine green and its application to hepatocellular carcinoma treatment" Journal of Cancer Metastasis and Treatment. 10: 16. http://dx.doi.org/10.20517/2394-4722.2024.18
ACS Style
Jo, E.; Savsani K.; Alfonso A.; Park A.; Lee SD.; Sakar D.; Kinsey N.; Sambommatsu Y.; Imaid D.; Khan A.; Sharma A.; Saeed M.; Kumaran V.; Cotterell A.; Levy M.; Bruno D. Photodynamic effect of indocyanine green and its application to hepatocellular carcinoma treatment. J. Cancer. Metastasis. Treat. 2024, 10, 16. http://dx.doi.org/10.20517/2394-4722.2024.18
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 0 clicks
Cite This Article 0 clicks















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.